Abstract

HMGB1, a key member of the HMG protein family, stabilizes nucleosomes and mediates infection, injury, and late inflammatory responses. Its related research is crucial in critical care medicine, cancer, and neurological diseases, yet current reports inadequately present its status. This review makes a comprehensive bibliometric analysis of the past hmgb1 research, and makes a graphical analysis of the trends and hotspots of HMGB1-related research. From 1976 to 2024, a total of 5992 articles were included, and the number of published papers and citations showed an increasing trend. China and the United States are leading contributors in this field, with the University of Pittsburgh being the most prominent research institution. Among the authors, Billiar, Timothy R. is the most prolific author, while Yang, H. ranks first in co-citation frequency. Inspection of the literature and keywords reveals that disease and its clinical diagnosis and treatment, cell death, mechanism, and molecular pattern are popular research topics. About disease frontiers, critical care medicine (sepsis, endotoxemia, severe viral hemorrhagic fever, multiple organ failure) is the earliest identified and very popular direction of HMGB1, and neurological diseases-related research are at the forefront of the field. In summary, this study employs bibliometrics to identify, visualize, and discuss trends and hotspots in HMGB1 research. Through comprehensive analysis of extensive literature, the aim is to clarify the current research status of HMGB1-related diseases, understand major trends, find potential collaborators, and discover promising and innovative research directions.
Keywords: HMGB1, disease, inflammatory responses
Introduction
1973 marked the first extraction and identification of the high mobility group (HMG) from calf thymus, the name of which derived from its high mobility in polyacrylamide gel electrophoresis[1,2] . As the most abundant HMG protein, the evolution of High Mobility Group Protein B1 (HMGB1) is highly conserved, which is also known as amphoterin[3] . HMGB1 is believed to be a nuclear protein that occurs in virtually all eukaryotic cells, binds loosely to chromatin, and serves to stabilize nucleosome formation[4-6] . It functionally like a transcription factor which regulates the encoding of multiple genes[7-9] . As research has progressed, it has become increasingly clear that when cells are subjected to stress, injury, or inflammatory stimuli, HMGB1 can be actively secreted or passively released into the extracellular environment, transforming it into a potent pro-inflammatory mediator[10] . In the first case, HMGB1 is released following acetylation in the nucleus during active modification and can be actively secreted by activated macrophages, mature dendritic cells, and activated N.K. cells[11,12] . This process is dependent on lipopolysaccharide-mediated (LPS-mediated) signaling via the TLR4-CD14 complex as well as TNF and TGF-β, which elicit HMGB1 migration from the nucleus and initiate the release of hyperacetylated HMGB1[13] . The second place is the passive departure of active HMGB1 from the cell, which frequently occurs during cell death, such as necrosis[10] . After its release, HMGB1 can transduce cellular signals by binding to at least three receptors: RAGE, TLR2, and TLR4. Signaling via RAGE activates the NF-κB signaling pathway, triggering cell survival and generating cytokines, including TNF, IL-6, and IFN-γ via signaling by ERK and p38[14] . TLR2 and TLR4 binding to HMGB1 activates the NF-κB pathway through MyD88-dependent mechanism[15,16] . These receptor-mediated signaling pathways can promote leukocyte chemotaxis, increase vascular permeability, and upregulate the expression of other pro-inflammatory factors, ultimately leading to enhanced inflammatory responses both locally and systemically.
In addition, HMGB1 has been shown to have a regulatory effect on the immune system[17,18] . Extracellular HMGB1, acting as one of the damage-associated molecular pattern (DAMP) molecules, can activate the innate immune system and has been recognized in recent years for its cytokine-like functions[19] . Under specific conditions, it also influences adaptive immune responses, such as by modulating the maturation of dendritic cells and the function of T cells. Therefore, it has been causally linked to a wide spectrum of diseases, including sepsis[20] , myocardial infarction[21] , atherosclerosis[22] , ischemia-reperfusion injury[23] , diabetes mellitus[24] , rheumatoid arthritis[25] , systemic lupus erythematosus[26] , liver[27] and pulmonary fibrosis[28] , SARS-CoV-2[29] , cancer[30] and neurodegeneration[31] .
Since the 1960s, with the popularization of the concept of open science, bibliometrics has ushered in remarkable development. The Internet has facilitated the open exchange and free sharing of scientific research results, and this revolution has had a profound impact on bibliometrics, from data analysis to support systems, to evaluation criteria and access to statistical information. By means of analyzing research in a specific field over a specific period, bibliometric analysis employs qualitative and quantitative methods, along with mathematical and statistical approaches[32] . This method focuses on disclosing the structure of countries, institutions, journals, authors, and keywords associated with research in that field, thereby presenting readers with an unbiased portrayal of trends and frontiers in the subject[33,34] . Bibliometric analysis has found utility across various research domains, such as innate immunity[35] , pyroptosis[36] , ferroptosis[37] , etc. Despite the recent exponential growth in HMGB1-related research, only limited efforts have been made to systematically assess the global scientific outcomes and status of this field.
As a result, a suitable visualization manner is urgently required to show the current state, potential trends, and hotspots of HMGB1-related research. Therefore, this study aimed to evaluate the overall picture of HMGB1-related research from 1976-2024 using VOSviewer, CiteSpace, and Bibliometrix (R-Studio's R-Tool) to identify the journals, institutions, and authors with the highest impact to enhance collaboration and learning. More importantly, these data will provide clinicians and researchers with future directions on the inflammatory response to HMGB1-related diseases to more fully present the current state of the field, potential trends, and hot spots.
Materials and Methods
Data source
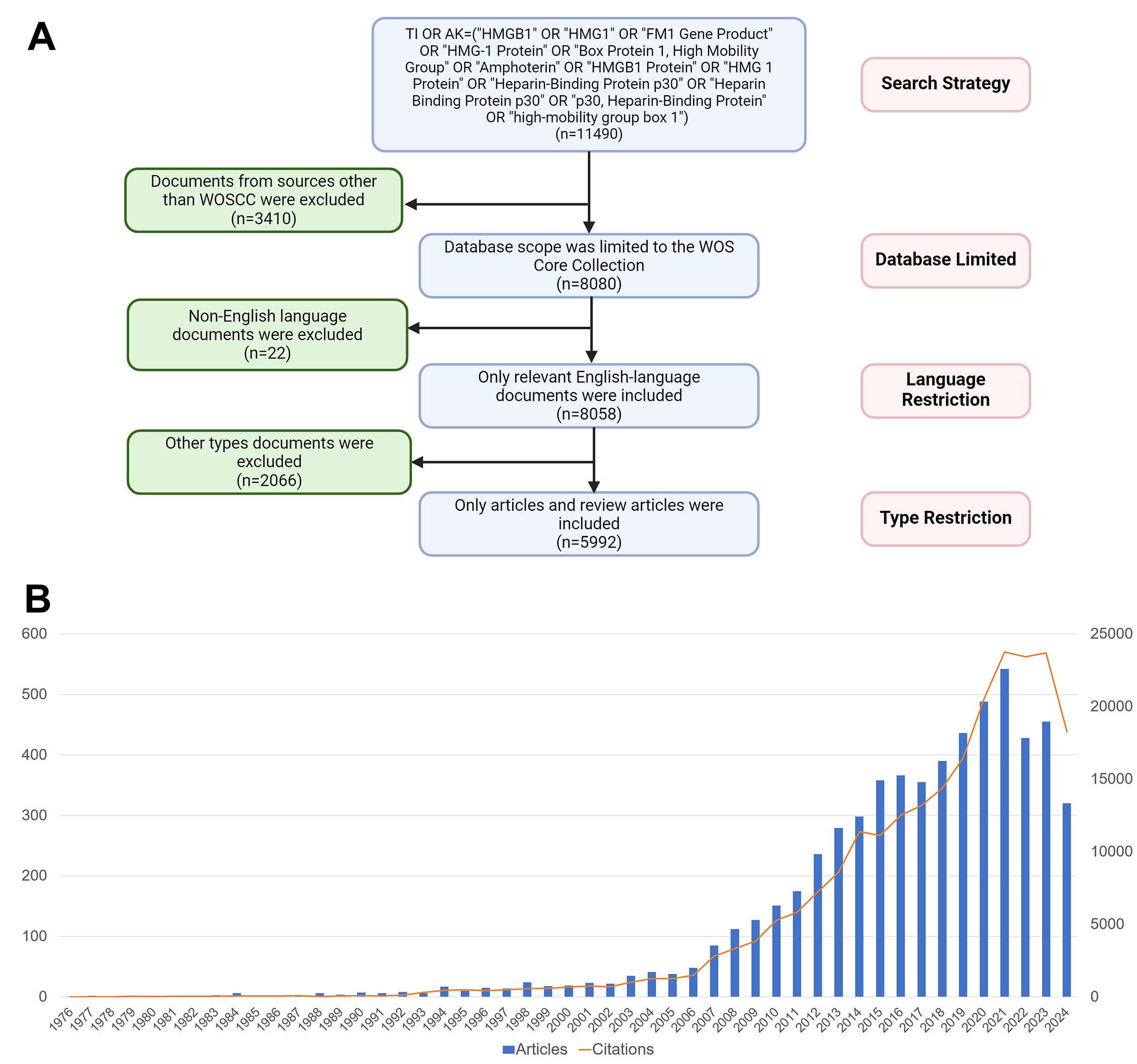
The data for the Bibliometrics in this study were sourced from the Web of Science Core Collection database (WOSCC), a standardized database that is frequently utilized in academia for scientific research and analysis. In WOSCC, T.I. denotes title, and A.K. denotes author keywords. The search strategy used in this research was TI=("HMGB1" OR "HMG1" OR "FM1 Gene Product" OR "HMG-1 Protein" OR "Box Protein 1, High Mobility Group" OR "Amphoterin" OR "HMGB1 Protein" OR "HMG 1 Protein" OR "Heparin-Binding Protein p30" OR "Heparin Binding Protein p30" OR "p30, Heparin-Binding Protein" OR "high-mobility group box 1") OR AK=("HMGB1" OR "HMG1" OR "FM1 Gene Product" OR "HMG-1 Protein" OR "Box Protein 1, High Mobility Group" OR "Amphoterin" OR "HMGB1 Protein" OR "HMG 1 Protein "OR "Heparin-Binding Protein p30" OR "Heparin Binding Protein p30" OR "p30, Heparin-Binding Protein" OR "high-mobility group box 1"). The search period is set to start from September 21, 1976 until November 7, 2024, with the inclusion criteria set to only Articles and Reviews, and the language confined to English. Ultimately, 5992 documents were subordinated in total. The results were exported in txt and xlsx format according to the above WOSCC search formula. In order to prevent data bias from database changes, the search was finished on November 7, 2024. Article citation analysis uses global citation counts, and co-citation analysis for countries, institutions, journals, keywords, etc., uses local citation counts from 5992 articles. The specific literature screening process is shown in Figure 1A.
Figure 1. (A) Flow chart of HMGB1-related research screening process. (B) Description of HMGB1-related publications and citations. The blue bar represents the number and trend of articles, and the orange line represents the number and trend of citations.
Data Analysis
For bibliometric analysis, CiteSpace, developed by Chaomei Chen, is now the most used program[38] . CiteSpace 6.1.R2 advanced visualization was utilized to examine international collaboration, journal dual map coverage, institutional distribution, topic area distribution, reference collaboration, and literature explosion. Bibliometric cooperation network graph analysis was the major use of VOSviewer developed by Nees Jan van Eck et al.[39] . Visual analysis of the distribution and collaboration among institutions, authors, journals, keyword collaboration and temporal evolution were performed using VOSviewer 1.6.18. Automatic clustering using VOS mapping techniques and similarity matrices is carried out, and subsequent addition of the corresponding labels is based on the content. With the help of Bibliometrix (an R-Tool from R-Studio), an open-source R package developed by Massimo Aria and Corrado Cuccurullo, we were able to visually analyze the annual average citation data, nation distribution, publishing collaborations, and reference collaborations[40] . Additionally, to visualize HMGB1-related publications and citation trends, we used Microsoft Excel 365.
Results
Annual publication and citation trends
From 1976, when the first article on HMGB1 was published, to 2024, the number of publications and citations in the literature relevant to HMGB1 are depicted in Figure 1A. The number of publications showed an overall upward trend, with a particularly noticeable increase in the number of articles after 2006. Among them, the most significant increase was from 2017 to 2021, with 542 articles published in 2021. Regarding the change in citations, the number generally showed an escalating trend year by year, with a significant increase in 2019-2021. Until 2021, the citations reached 23749, with the highest annual growth rate of 15.91%.
Inter-country distribution
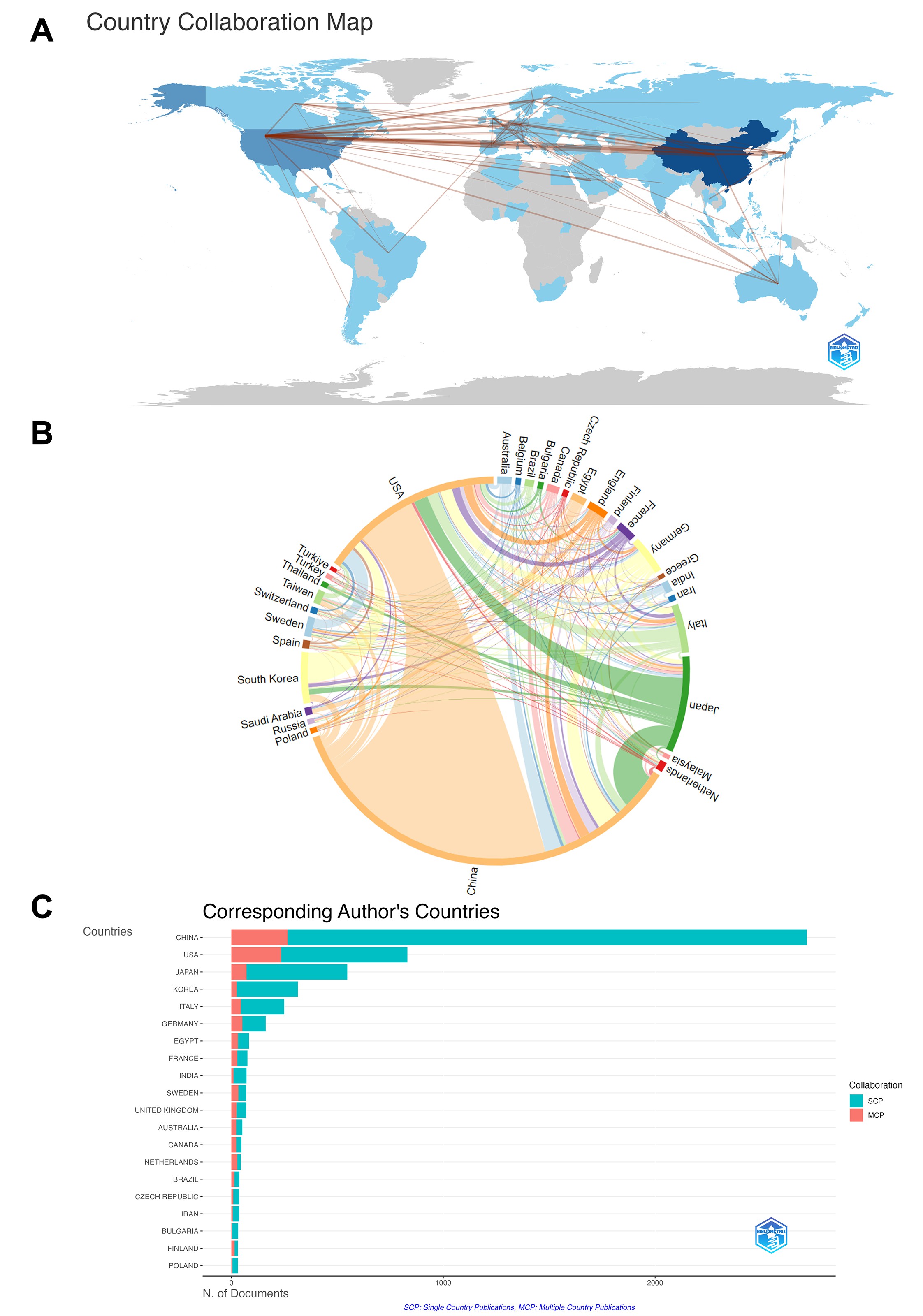
As of November 7, 2024, 66 countries or regions have published a total of 5992 HMGB1-related studies (Supplementary Table 1). In terms of the number of publications, China is the first (2759), followed by the United States (1202) and Japan (626), with the account of China and the United States which is more than half of all. Regarding citations, the United States ranked first (95984), followed by China (68067) and Italy (33038), displaying an obvious distinction from the rest countries. In terms of total link strength, the United States is the first (743), followed by China (427) and Germany (196). Vertically, among the top ten countries, the publications of top 2 are above 1200 while the rest are under 700, and the citations of top 2 are above 60000 while the rest are under 40000, showing a significant gap.
International collaborations for HMGB1-related research are shown (Figure 2A), mainly concentrated in the northern hemisphere, with collaborations between the United States and Europe, the United States and China, and the United States and Japan dominating. The chord diagram shows the collaborations between countries or regions (Figure 2B). The total number of publications from the United States and China accounted for 66.1% of HMGB1-related studies. The cooperation between countries was dominated by the United States and China, followed by collaborations between the U.S. and Japan, Germany, and Italy. In terms of the ratio between domestic and international collaborative publications (Figure 2C), most European countries such as the Belgium (62.5%), Sweden (47.8%), United Kingdom (34.8%), France (35.5%) have a higher share of international collaborative publications, while the United States (28.2%) and Italy (18.1%) has a balanced development, and Japan (13%), China (9.8%), and Korea (8.0%) are relatively low.
Figure 2. (A) Countries/regions collaboration map. On the map of world administrative divisions, the lines between countries/regions represent collaborations, and more lines mean more frequent collaborations. (B) The chord diagram of collaboration between countries/regions. The arc is proportional to the total number of publications, the width of the arc is proportional to the total number of publications, and the width of the shading is proportional to collaborative publications between the two countries. (C) Analysis of the corresponding author's country. Blue represents the number of single country publications (SCP) and red represents the number of multiple collaborative publications (MCP). (Country analysis includes nations with a publication volume greater than 25.)
Institutional distribution
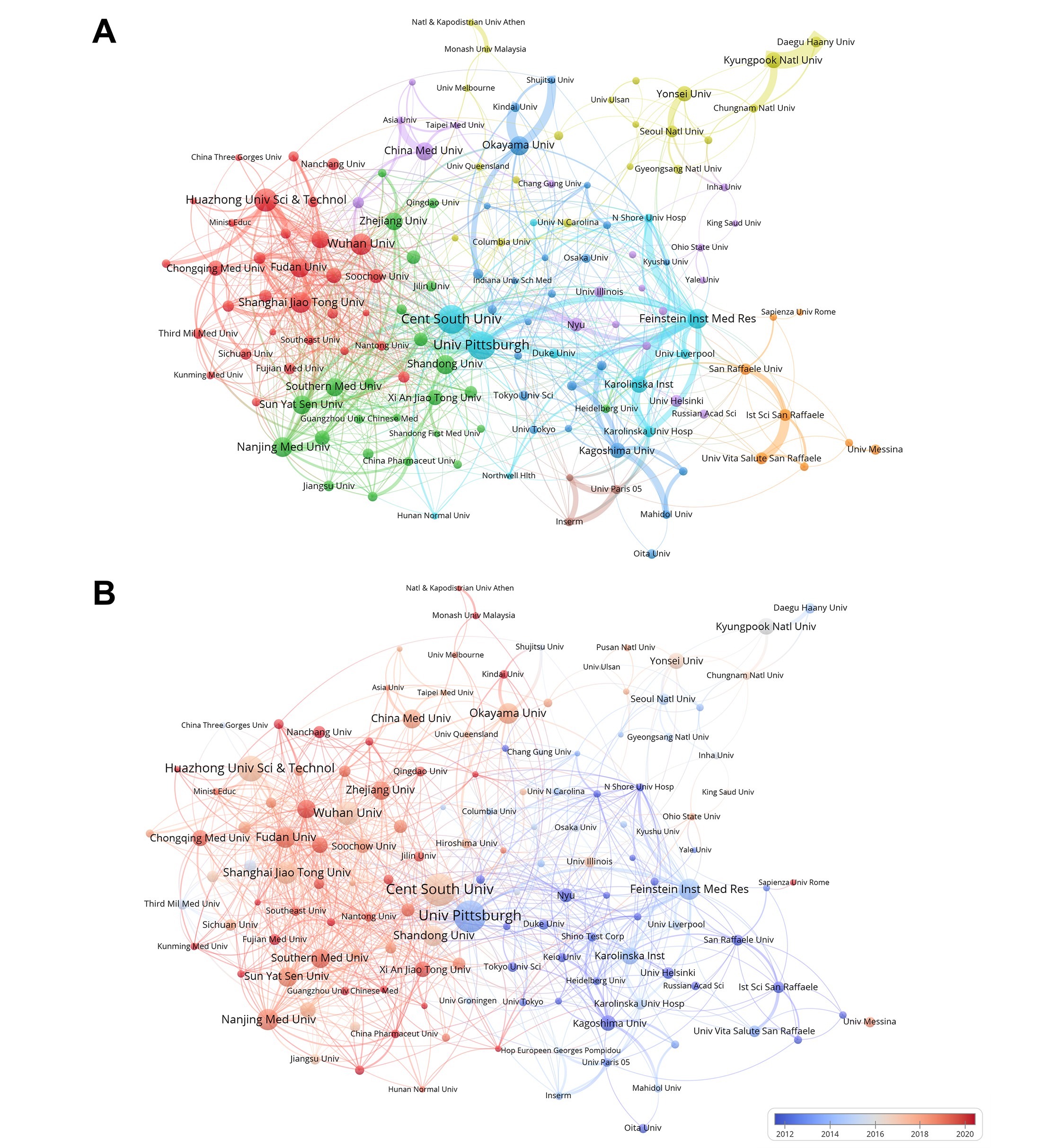
Supplementary Table 2 lists the top 10 institutions of publications, citations, and total link strength. In terms of publications, 7 of the top 10 institutions are in China, followed by 2 in the United States and 1 in Japan. The institution with the highest number of publications is Cent South Univ (190), followed by Univ Pittsburgh (185), and Huazhong Univ Sci & Technol (133). The most cited institution is under Univ Pittsburgh (23383) and Feinstein Inst Med Res (14480) in the U.S., followed by Ist Sci San Raffaele (10742) in Sweden. In terms of total link strength, it is still Univ Pittsburgh (157), Feinstein Inst Med Res (140) in the U.S., and Cent South Univ (139) in China are in the lead. Longitudinally, among the top ten institutions in terms of publications, the highest two are more than 180, followed by the third are less than 140; in terms of citations, there is a clear gap between the top (both more than 23000) and the bottom (all less than 5000). From a horizontal perspective, Univ Pittsburgh is at the top of both publications and citations, which implies that the institutions have an important position in research in HMGB1-related fields. Notably, the number of Feinstein Inst Med Res publications did not even make the top 5, but the number of citations was significantly high, which proves the outstanding quality of the research published at this institution in the field of HMGB1.
We used VOSviewer to cluster the inter-institutional collaborations into eight closely related clusters (Figure 3A). The cluster analysis of these research institutions aimed to understand global distribution and collaboration. Overall, institutions within clusters cooperated closely showed a balanced distribution, such as China (red, e.g., Huazhong Univ Sci & technol, Wuhan Univ), American (light blue; e.g., Feinstein Inst Med Res, Univ Pittsburgh), France (brown; e.g., Univ Paries, Inserm), Korea (yellow; e.g., Kyungpook Natl Univ, Yonsei Univ), and Japan (dark blue; e.g., Kagoshima Univ, Okayama Univ). In contrast, in the United States, HMGB1-related research has the characteristic of radiating outward mainly from Univ Pittsburgh, Feinstein Inst Med Res. It is worth noting that the cluster (light blue; e.g., Univ Pittsburgh, Cent S Univ) does not belong to a single country, and the University of Pittsburgh and Cent S Univ included in it play an essential role in linking collaborative research publications from multinational research institutions. Figure 3B presents a visual analysis of the institutional affiliations and their associated collaborative networks pertaining to articles in the field from 2012 to 2020. Red nodes indicate institutions that have been more active recently, such as Fujian Med Univ, Nanchang Univ, and Kunming Med Univ, highlighting their forefront positions in recent HMGB1-related research. Blue nodes represent institutions that were more active in the past, including Kagoshima Univ, San Raffaele Univ, and NYU. As one of the core nodes, Univ Pittsburgh has published a significant number of papers and has collaborated closely with many other institutions.
Figure 3. (A) Analysis of HMGB1-related collaborative network visualization of institutions in VOSviewer. Vosviewer automatically clusters all institutions into different colored clusters based on inter-institutional collaboration. Each dot represents an institution, the size of the dot reflects the number of publications, and the thickness of the line between the dots reflects the closeness of the collaboration between institutions. (B) Institutional analysis of published articles from 2012 to 2020. (Institutional analysis includes organizations with a publication volume greater than 15.)
Author Distribution
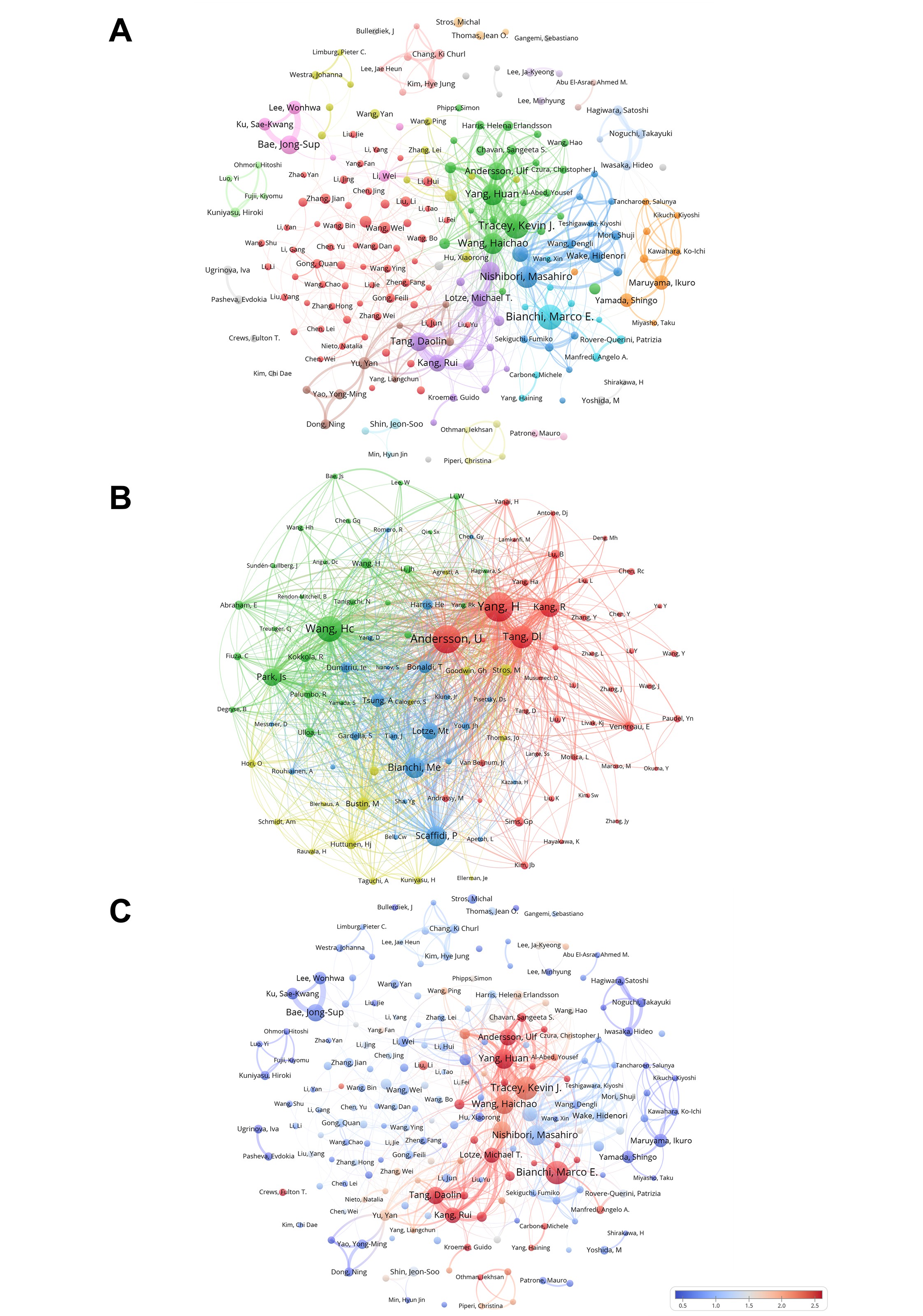
By analyzing the authors of the sample reference, we listed the top 10 authors in publications and co-citations (Supplementary Table3) to display the research strengths of the authors and the research hotspots related to HMGB1. The most published author was Bianchi, Marco E., followed by Tracey, Kevin J., and Nishibori, Masahiro. The most co-cited was Yang, H, followed by Andersson, U, and Wang, Hc.
The collaboration between authors is shown in Figure 4A, which can guide the search for research partners and the judgment for authoritative institutions in this field. The results of the VOSviewer cluster analysis indicate that collaborations among Tracey, Kevin J., Yang, Huan, Wang, Haichao, is are significantly close. Interestingly, the collaboration among authors seems to exhibit a geographical clustering pattern, with affiliations broadly reflecting their respective countries or regions. For instance, green (the United States), light blue (Europe), brown and red (China), light purple (Japan), and orange (South Korea). The co-cited authorship network map using VOSviewer (Figure 4B) shows that the citation profile of HMGB1-related studies is pronounced clustering, forming four major clusters: the red cluster mainly includes Yang, H, Andersson, U, Tang, Dl, Kang, R, the blue cluster mainly includes Bianchi, Me, Scaffidi, P, the green cluster mainly includes Wang, Hc, and the yellow clusters, in which internal distribution is relatively balanced. Figure 4C presents an analysis of the popularity of articles published by various authors over the past five years. The heat value for each author is calculated by dividing the number of papers they have published in the recent five years by their total number of publications. The results indicate that the works of Bianchi, Marco E., Yang, Huan, Andersson, Ulf, and Tang, Daolin, among others, are highly popular among readers. In contrast, the popularity of articles authored by Bae, Jong-Sup, Yamada, Shingo, and Maruyama, Ikuro has relatively declined during this period.
Figure 4. (A) Analysis of HMGB1-related collaborative network visualization of authors in VOSviewer. VOSviewer divides authors into different colored clusters based on their collaboration status. Different colors represent closer collaboration relationships. Each dot in the graph represents an author, the size of the dot reflects the number of publications of the author, and the thickness of the line between the dots reflects the degree of cooperation. (B) Analysis of collaborative network visualization of co-cited authorship in VOSviewer. The analysis relates to one or more future works simultaneously citing two or more authors. Higher co-citation frequency denotes more concentrated academic attention and research. Vosviewer automatically clusters authors into different colored clusters based on their co-cited status, where each dot represents an author, the size of the dot reflects the co-cited strength, and the thickness of the line between the dots reflects the co-cited relationship of the two authors. (C) Analysis of authors that publish articles. The heat value for each keyword over the past five years is calculated by dividing the number of publications in the last five years by the total number of publications. (Author analysis includes authors with a publication volume greater than 10. Co-cited author analysis includes authors with citation counts greater than 175.)
Journal Distribution
The top 10 journals in terms of publications and citations were compiled by taking into account the journals that have been published in the literature on HMGB1 (Supplementary Table 4). The table's impact factor (IF) and Journal Citation Reports (JCR) quartiles show how influential the journals are. International Immunopharmacology (104; 4.8, Q1), International Journal of Molecular Sciences (160; 98, Q1), Plos One (92; 2.9, Q1), Frontiers in Immunology (88; 5.7, Q1), and Biochemical and Biophysical Research Communications (87; 2.5, Q3) are the journals with the most publications. Among the top ten journals, seven journals are in the Q1 division, and five of them have an IF above 4. These data indicate that HMGB1 is highly regarded and influential, in which results are primarily published in well-known journals. Journals with the most co-citations are J Biol Chem and J Immunol. The Q1 division has seven of the top ten journals by co-citations, and three of these journals had an IF of more than 40. Notably, International Immunopharmacology and Journal of Biological Chemistry rank among the top journals for publications and co-citations, demonstrating their authority in the field of HMGB1.
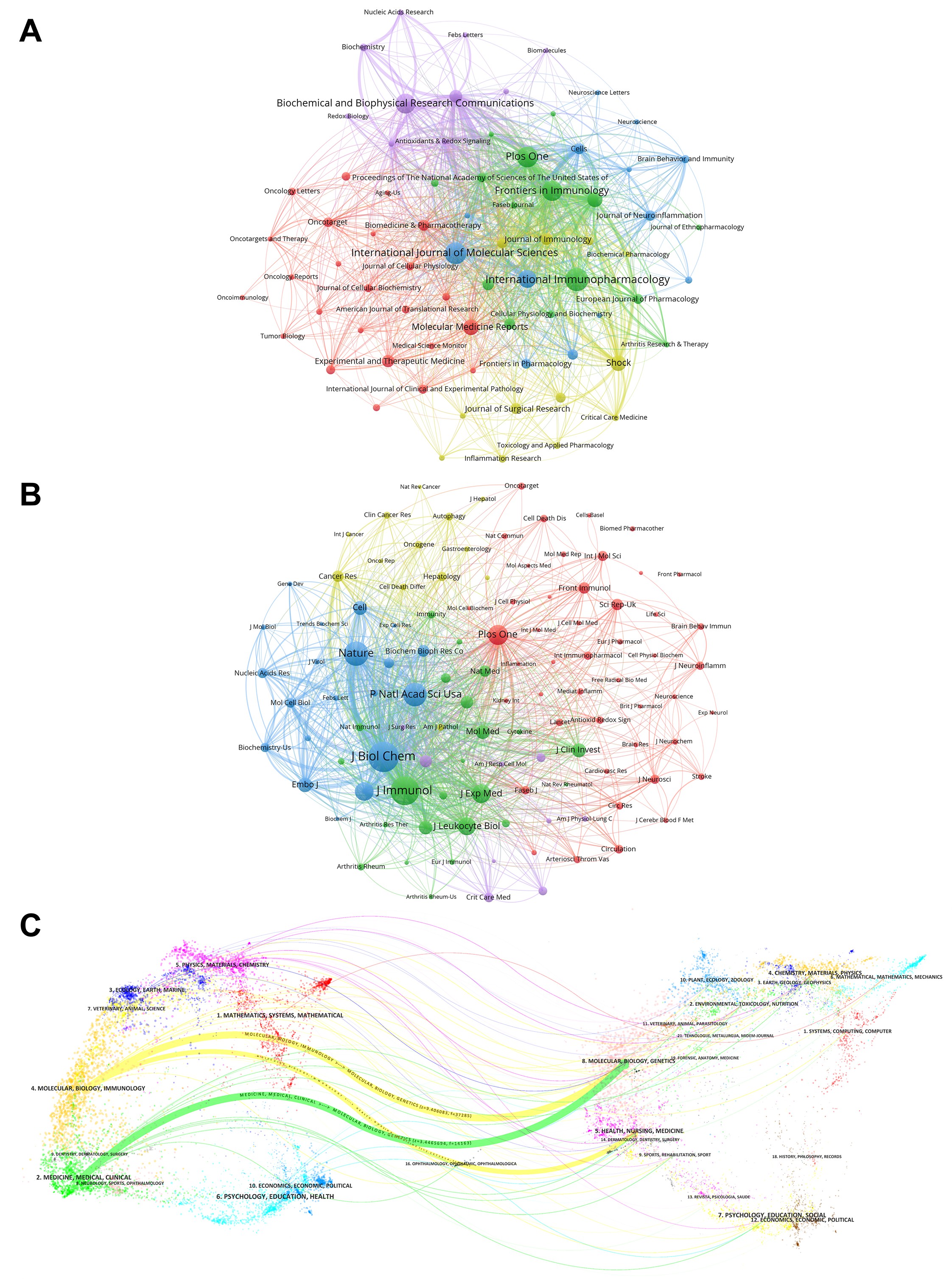
In Figure 5A, all of the journals are generally divided into five clusters based on publications: The red cluster is concerned with cancer and its treatment (Biomedicine & Pharmacotherapy, Oncotarget, Oncoimmunology, etc.); the green cluster is concerned with immune function and cell biology; the yellow cluster is concerned with critical care medicine (Shock, Critical Care Medicine); and the blue cluster is concerned with neurology (Journal of Neuroinflammation, Neurosciance, etc.); the purple Cluster focuses on Biochemistry (Biochemistry and Biophysical Research communications). In Figure 5B, all of the journals were grouped into five clusters based on the co-citations, which have a propensity for sharing research trajectories. The red cluster focuses on blood-related fields (Circulation, Arterioscl Throm Vas, J Cerebr Blood F Met, etc.); the green cluster favors various clinical diseases such as immunology (J Immunol, J Clin Invest, etc.); the yellow cluster mainly deals with cancer, cell death (Cancer Res, Oncogene, Cell Death Differ, Autophagy, etc.); the blue cluster focuses on cell biology (J Biol Chem, Cell, Mol Cell Biol, etc.); the purple cluster involves critical care medicine (Crit Care Med, etc.). Through knowledge flow analysis, we investigated how published and cited journals relate to one another, which is displayed on the dual-overlay journal map (Figure 5C). The most frequently published journals are those from forefront fields including MOLECULAR, BIOLOGY, IMMUNOLOGY, NEUROLOGY, SPORTS, OPHTHALMOLOGY, MEDICINE, and MEDICAL CLINICAL. The majority of the referred journals are from authoritative information sources like MOLECULAR, BIOLOGY, GENETICS, HEALTH, NURSING, MEDICINE, DERMATOLOGY, DENTISTRY, and SURGERY. Notably, the most frequently published and most commonly cited journals overlap highly, which means that these journals are at the forefront of HMGB1-related research.
Figure 5. (A) Analysis of HMGB1-related collaborative network visualization of journals in VOSviewer. The visualization in VOSviewer displays the journals and their relationship based on the proximity of the journals. Different dots represent different journals, the color reflects the corresponding clusters, and the connecting line indicates the closeness of the connection. (B) Analysis of collaborative network visualization of journal citations in VOSviewer. Different dots represent different journals, the colors reflect the corresponding clusters, and the lines indicate the closeness of the connections. (C) The dual-map overlay of journals. The distribution of themes, variations in citation trajectories, and changes in research centers are all displayed. The published journals are labeled on the left side of the double map, while the cited journals are labeled on the right. The context of the citation connection is indicated by the colored arrows pointing from the published journal to the referenced journal. (Journal analysis includes journals with a publication volume greater than 15. Co-cited journal analysis includes journals with citation counts greater than 500.)
Keyword analysis
The principal themes of the article are encapsulated by its keywords, and the analysis of keyword co-occurrences can be employed to evaluate the developmental status of research pertaining to HMGB1. We listed the top 10 keywords by frequency of occurrence (Supplementary Table 5). Except for "HMGB1" (3990), the keyword with the highest frequency is "inflammation" (758), followed by "RAGE" (the Receptor of Advanced Glycation Endproducts, 379), "sepsis"(305), and "toll-like receptor 4"(257), which indicates that they are hot issues in the field of HMGB1 counterpart research.
A co-occurrence network map of keywords is displayed in VOSviewer (Figure 6A). According to the concerned areas, the keywords were grouped into seven categories, based on the obtained results. The red cluster includes the topics covered by cancer and cell death (breast cancer, NSCLC, hepatocellular carcinoma, autophagy, apoptosis, migration, metastasis). The blue cluster is related to neurological damage (neuroinflammation, epilepsy, stroke, microglia). The green cluster is related to acute injury and oxidative stress (sepsis, acute lung injury, oxidative stress, cytokine). The yellow cluster is associated with cardiovascular disease (atherosclerosis, myocardial infarction, and angiogenesis); the purple and cyan sections are related to mechanisms of immune diseases (rheumatoid arthritis, endothelial cells, dendritic cells, innate immunity, and diabetic nephropathy). The orange cluster mainly involves inflammatory molecules (Toll-like receptor, IL-6; TNF-α). Attentionally, the cyan cluster (immunity) is extensively connected to all other clusters, as it involves nodes that are key focuses in HMGB1 research.
Figure 6B shows the Thematic evaluation map of related research, which visualizes four different periods of themes according to two dimensions (density and centrality). In terms of motor themes (Q1), from 1990 to 2010, the focus was primarily on foundational studies of molecular structures, such as DNA bending, mRNA localization. Entering the period from 2010 to 2015, the interest shifted towards inflammatory factors and cell types, including damage-associated molecular patterns (DAMPs), alarmins, and dendritic cells. Subsequently, between 2015 and 2022, the research emphasis expanded to encompass diseases closely linked with the immune system, particularly focusing on hepatocellular carcinoma, rheumatoid arthritis, and neurological disorders. In the most recent phase, from 2022 to 2024, there has been an increased concentration on exploring neurological conditions (neuroinflammation and microglia functions) along with DAMPs, hypoxia, and proliferation. Regarding niche topics (Q2), these involve diseases that are less studied (diabetic nephropathy, melanoma) and molecular mechanisms (immunogenic cell death, vascular endothelial growth factor, in situ hybridization). For emerging or declining topics (Q3), these include fibrosis, asthma, thrombomodulin, acetylation, and other themes that may develop in the future or are currently in decline. In basic themes (Q4), sepsis and pyroptosis have been relevant to this area over the past two years but have not yet been fully developed. They are expected to become future hotspots (Figure D).
Figure 6. (A) Analysis of HMGB1-related collaborative network visualization of keywords in VOSviewer. Clusters are formed based on keyword co-occurrence frequency, with colors distinguishing clusters. Node size indicates keyword frequency, and line thickness represents relationship strength. (Keyword analysis includes terms that appear more than 20.) (B) Thematic evaluation of the research field during 1990-2010. (C) Thematic evaluation of the research field during 2010-2015. (D) Thematic evaluation of the research field during 2015-2022. (E) Thematic evaluation of the research field during 2022-2024. (B-E) The thematic evaluation map visualizes four different periods of themes according to two dimensions (density and centrality). Motor themes are the keywords in the upper right corner of the first quadrant. Niche themes are located in the upper left (second quadrant), emerging or declining themes are in the lower left (third quadrant), and basic themes are in the lower right (fourth quadrant). The Motor Themes in the upper right quadrant represent directions that are relevant and already rapidly developing in the field of HMGB1, which is the focus of current research. The Basic Themes in the lower right quadrant represent topics that are relevant to the field of HMGB1 but are not yet well developed and may be the focus of future research in the field of HMGB1.
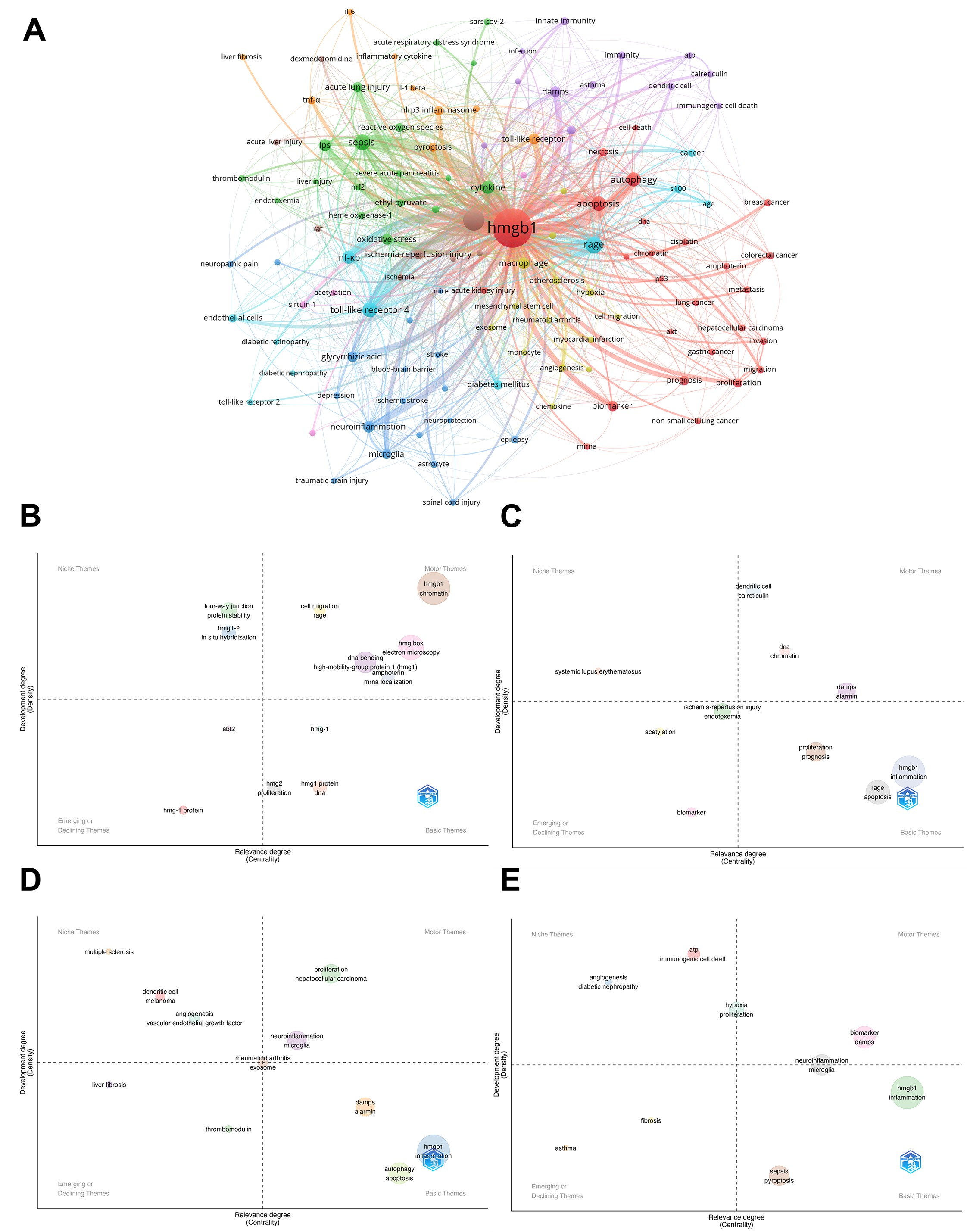
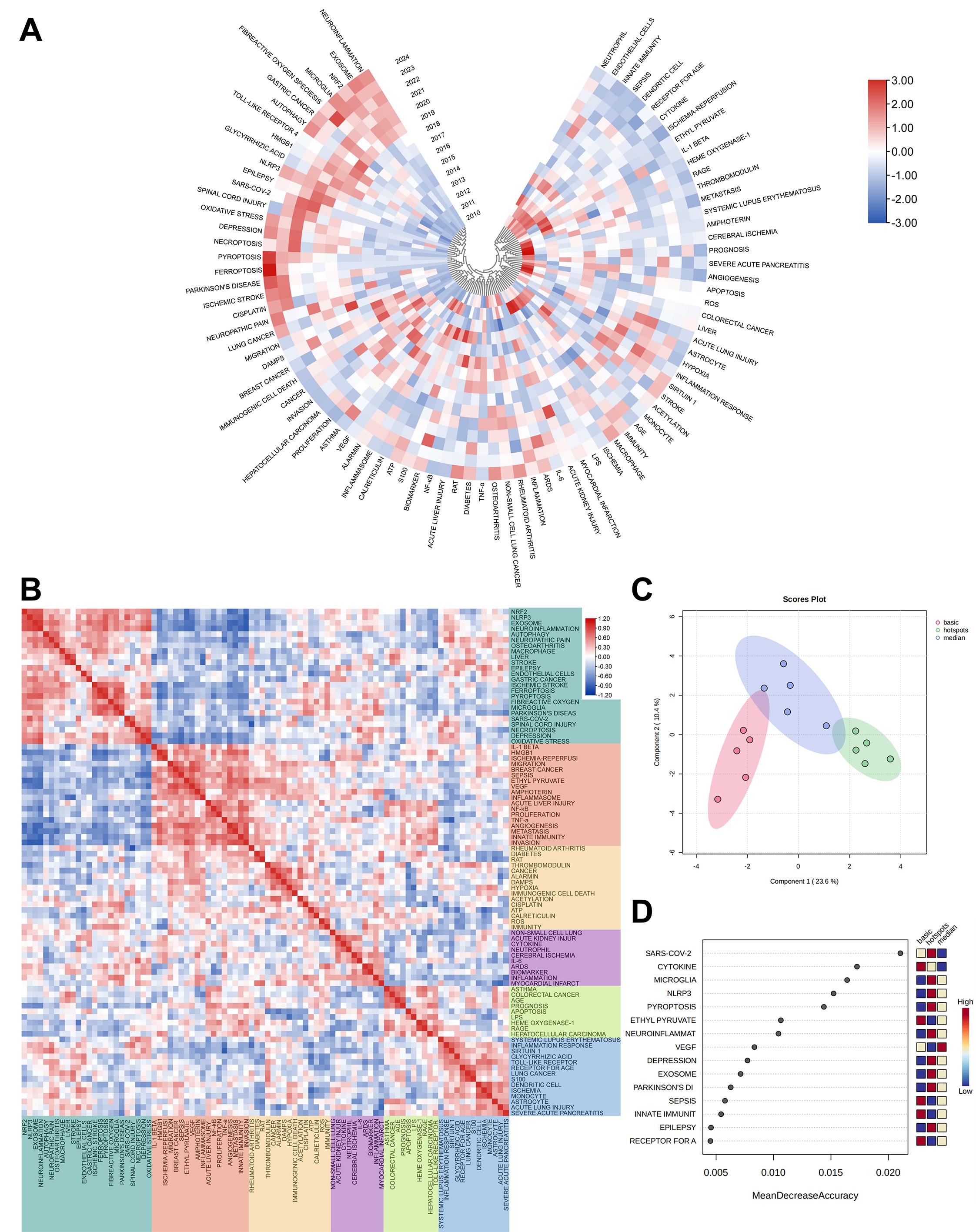
Figure 7A extends the keyword analysis by adding a temporal layer, illustrating that research before 2019 was mainly concentrated on critical illnesses such as HMGB1-associated malignancies, sepsis, myocardial infarction, and cerebral ischemia as. Since 2019, the research has branched into three distinct streams: firstly, HMGB1-related receptor proteins and active components, such as NLRP3, NRF2, glycyrrhizic acid, and toll-like receptor 4; secondly, neurological conditions such as depression, epilepsy, ischemic strokes, spinal cord injuries, and neuroinflammation; and thirdly, the modes of programmed cell death include autophagy, as well as recent research hotspots such as ferroptosis and pyroptosis. Figure 7B analyzes and depicts a heatmap of the co-occurrence frequencies among the top 100 keywords. Pronounced red areas indicate strong correlations between keywords such as necroptosis, ferroptosis, and neuroinflammation, which are highly related to spinal cord injury, depression, epilepsy, and exosomes. Systemic lupus erythematosus and severe acute pancreatitis are linked to immune responses, sirtuin 1, S100, toll-like receptor and monocyte. Crucially, HMGB1 emerges as the keyword with the highest frequency of occurrence, predominantly connected to critial care medicine such as sepsis, ischemia-reperfusi, acute liver injury, and breast cancer, with implicated mechanisms involving innate immunity, TNF-α, NF-κB, cytokines, amphoterin, and ethyl pyruvate. To better define the temporal classification of these keywords for trend analysis, we manually categorize them into three groups: basic (prior to 2004), median (2005–2014), and hotspots (2015–2024). The results of the principal component analysis (PCA) (Figure 7C) indicate that while the "intermediate" group partially overlaps with the other two groups, there is a clear separation between the groups. This suggests that the internal similarity within each group has changed significantly over time, validating the effectiveness of the classification and indicating substantial changes in the evolution of keywords in this field. Subsequently, the analysis of keyword heat using the random forest method (Figure 7D) shows that the keywords with higher recent heat values include SARS-CoV-2, microglia, NLRP3, pyroptosis, neuroinflammation, depression, exosome, Parkinson's disease, and epilepsy.
Figure 7. (A) Heatmap of keywords in temporal dimension. Each radial line corresponds to a specific keyword, while each concentric circle denotes a successive year. A color block with a more intense red indicates a higher frequency of the keyword's occurrence in that year's literature. (B) Heatmap of the top100 keywords. It depicts a keyword heatmap where each cell's color intensity correlates with the co-occurrence frequency of the two respective keywords it represents. A deeper red hue within a cell signifies a higher co-occurrence rate, indicating that extensive red areas are often indicative of a high degree of relevance between the associated keywords. (C) Annual heat map spanning from 1976 to 2024. The annual heat value for each keyword is calculated by dividing the citation count for that year by the total citations made in the same year. (D) Heat map illustrating keyword correlation. Keywords with high visibility are divided into different categories and distinguished by different colors.
Analysis of critical literature and references
We listed the top 15 cited references (Supplementary Table 6). The most highly cited reference is "Release of chromatin protein HMGB1 by necrotic cells triggers inflammation"[41] . It proposes that necrotic cells signal the surrounding environment by releasing HMGB1 and activate the inflammatory response by binding to receptors such as RAGE, revealing the dual role of HMGB1 in the cell nucleus and its involvement in the inflammatory process as a signaling molecule in the extracellular environment. The second highly cited reference is "Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein"[42] . This study investigates how HMGB1 enhances the nuclear translocation of NF-κB and the expression of pro-inflammatory cytokines by activating Toll-like receptors 2 and 4 (TLR2 and TLR4), thus playing a role in acute inflammatory responses. In comparison, RAGE has a more limited role in HMGB1's activation of macrophages, and the response it elicits differs from that caused by lipopolysaccharide (LPS).
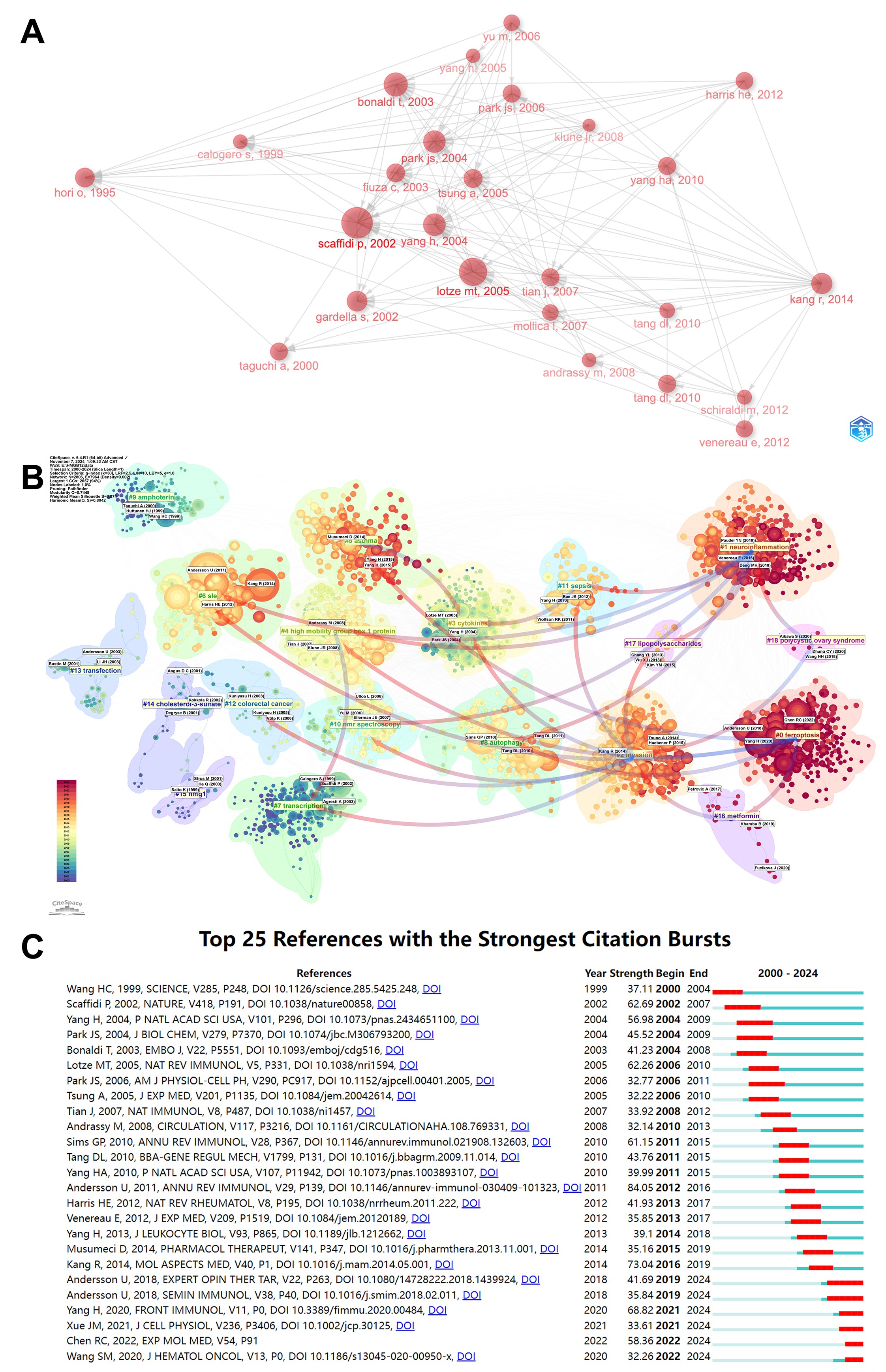
Figure 8A shows the citation relationship between the top 25 documents. The results show that "Release of chromatin protein HMGB1 by necrotic cells triggers inflammation", published by Scaffidi, P et al. in 2002 received the most citations of other articles, which firstly reported HMGB1 released from necrotic cells promotes inflammation by signaling cell death to neighboring cells. In contrast, apoptotic cells are programmed to retain the signals that would otherwise be spread by damaged or dying cells, thus they do not release HMGB1 and consequently do not promote inflammation. Subsequently, "Extracellular role of HMGB1 in inflammation and sepsis." published by Yang, H et al. 2004, "Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein" published by Park, JS et al. 2004 and "Nuclear factor HMGB1 mediates liver injury after murine liver ischemia-reperfusion" published by Tsung, A et al. 2005, were the most cited articles, which served as a bridge between the previous stage and the next stage. Finally, "HMGB1 in health and disease" by Kang et al. 2014 cited almost all of the articles in Figure 8A, providing a comprehensive review of HMGB1-related research.
Through CiteSpace, we analyzed the relationship between co-citations among the literature in the field of HMGB1 (Figure 8B). The clusters were divided into 18 categories and shown in various colors. Cluster #0 is involved in Ferroptosis. According to publication years of literature, we observed the development of HMGB1-related study from the time dimension. The earliest areas of HMGB1-related literature from 1999-2003 were #9 (amphotericin), #15 (HMG1), #14(cholesterol-3-sulfate) and #13 (transfection), a period in which the infrastructure and binding sites of HMGB1 itself was mainly explored. 2004-2010, studies focused on #12 (colorectal cancer), #10 (NMR spectroscopy), #3 (cytokines), and #4 (high mobility group box 1 protein). In 2010, the number of studies on the relevance of autophagy, invasion and HMGB1 proliferated, and then emerged #8 (autophagy), #6 (systemic lupus erythematosus), #5 (asthma), #11 (sepsis), #2 (invasion), etc. In 2015 and beyond, the intensity between studies decreased, with the emergence of several clusters: #1 (neuroinflammation), #0 (ferroptosis), #16 (metformin), and #18 (polycystic ovarian syndrome). It is worth noting that the #1 (neuroinflammation) cluster has the most significant number among those clusters.
Figure 8C shows the top 25 references with the strongest citation bursts. The initial citation bursts occurred in 2000. The title of the article is "HMG-1 as a Late Mediator of Endotoxin Lethality in Mice." Notably, "HMGB1 Is a Therapeutic Target for Sterile Inflammation and Infection" by Andersson, Ulf et al. in ANNU REV IMMUNOL 2011 was the most explosive paper (intensity = 84.05), followed by " HMGB1 in health and disease" by Kang, R et al. in MOL ASPECTS MED 2014 (intensity = 73.04). These two high-outbreak review articles also illustrate the authoritative status of Andersson, Ulf, and Kang, R in the field of HMGB1-related research. 2015 and beyond, the outbreak continues until 2022, mainly involving new molecular patterns such as Caspase dependence of HMGB1 in driving the inflammatory process, and in infections, endotoxemia, and other inflammatory diseases as well as cancer, targeting the secretion and release of HMGB1 provides effective therapeutic strategies, suggesting that these directions still have a good scope for development.
Figure 8. (A) Association between the top highly cited references. Each node represents a highly cited paper, and arrows indicate citation relationships, pointing from the citing paper to the cited paper. The size of the nodes is proportional to the number of co-citations the paper has received. (B) Clustering of references based on the similarity between references, which shows the authors and the year of publications with an explosive increase in co-citations. The category with the most articles is 0, followed by 1, and so forth. (C) The top 25 references with the strongest citation bursts. The blue line indicates the timeline, and the red segments represent burst periods. A citation burst represent a sharp increase in the number of articles cited and some of the key issues in the field raised or addressed in the article.
Discussion
The analysis in this study is based on 5992 HMGB1-related papers from 66 countries/regions that were archived in the WOSCC database from 1976 to 2024. The 5992 HMGB1-related literature was analyzed using CiteSpace 6.1.R2 Advanced, VOSviewer 1.6.18, and Rbibiometrix. Based on these outcomes, the region's geographic and temporal distribution, author contributions, core articles, research hotspots, and field frontiers were evaluated.
General Distribution
In the general trend analysis, the rapid increase in publications and citations indicates that HMGB1 is receiving increasing attention. Over the past 15 years, the quantity of HMGB1-related studies has continuously increased, with the most significant growth occurring between 2017 and 2021. By 2021, the number of HMGB1-related publications will be approximately ten times higher than in 2004. This substantial rise is largely attributed to the emergence of the COVID-19 pandemic, which sparked considerable academic interest in the role of HMGB1 in pulmonary inflammatory diseases. The overall trend in the number of publications indicates a steady growth of research. Attentionally, a sudden increase of the citation index emerged in 2021-2022 which means a possible breakthrough in the area. The total link strength and the number of publications are two key metrics in the country/region analysis. These nations/regions with high total link strength serve as "bridges" in the global cooperation network. Horizontally, China and the United States are in the top either publications or citations, which proves their strong overall strength. It is noteworthy that Italy and Germany have a high number of citations despite the low number of publications, which proves the high quality of research published in the field in these countries.
In the examination of research institutions, seven of the top ten institutions in terms of publications are from China, followed by two in the United States and one in Japan. The United States leaders in terms of publication, citation, and total link strength are Univ Pittsburgh and Feinstein Inst Med Res. In addition, research institutions from China, Sweden, Germany, Italy, France, Japan, and Korea are also widely involved in HMGB1-related collaborations. In the author analysis, as shown in Supplementary Table 3, Yang, H, and Andersson, U ranked first in co-citations, far exceeding other scholars. It is also evidenced by 4 of the 25 highest outbreak literature published by Yang, H and 3 by Andersson, U., demonstrating their tremendous influence in HMGB1-related fields. In addition, Andersson, U (second place in co-citations) has co-authored several high burst articles with Yang, H. (first place in co-citations). Interestingly, Andersson, U ranked second in terms of co-citations but was only top 9 publications, probably because his research focus is on inflammation rather than specifically on HMGB1. The quality of its early publications at the intersection of these two fields is authoritative, with a gradual citation blast. Inferred from the central position within the collaboration map, Tracey, Kevin J., Wang, Haichao, and Yang, Huan are productive authors. In the co-citation author network, due to the multi-disciplinary and multi-field nature of HMGB1 research, authors from different clusters also maintain close collaborative relationships. Kang, R., from the red cluster, along with several prominent authors from other clusters, comprehensively summarized the structure, function, regulatory mechanisms, and roles of HMGB1 in various diseases, particularly in inflammatory conditions such as sepsis. They also explored the potential of HMGB1 as a biomarker and discussed therapeutic strategies targeting HMGB1, providing valuable references and directions for future research and clinical applications[43] .
Among the top 10 journals, journals with high volumes of publication and co-citations include PLoS ONE and the Journal of Biological Chemistry. Among them, Journal of Biological Chemistry had the most co-citations but only the top 10 publications, principally because it comprised a great deal of pertinent and highly cited papers. Notably, the majority of publications in this research field cover a broad spectrum of disciplines, including immunology, neurology, sports science, ophthalmology, dermatology, nursing, and clinical medicine. However, recent studies on HMGB1, particularly in the context of neuroinflammation within neurology, have become increasingly in-depth, suggesting that HMGB1 in neurology will be a research hotspot in the coming period.
The analysis of the top ten co-cited references reveals their profound impact on subsequent research in the HMGB1 field. The groundbreaking study by Scaffidi, P., et al. reshaped our understanding of sterile inflammation and cell death signaling. This discovery spurred transformative research into HMGB1's dual nuclear/extracellular roles and its pathological implications in autoimmune diseases (SLE), cancer (glioblastoma chemoresistance), and ischemia-reperfusion injuries. This paper experienced a significant burst in citations between 2002 and 2007, significantly influencing subsequent studies. Another influential work by Park, JS, et al. demonstrated that HMGB1 activates TLR2 and TLR4 to enhance NF-κB-driven pro-inflammatory cytokine release, providing a theoretical basis for studying disease mechanisms across various conditions and developing therapeutic strategies. However, the multifunctionality of HMGB1 and the complexity of its signaling networks remain key challenges and focal points in current research. Future studies need to integrate multi-omics technologies and clinical translational research to resolve mechanistic controversies and achieve precise interventions.
Hot spots and frontiers
Keyword analysis and citation analysis are helpful in understanding the cutting-edge and hot topics within the HMGB1 field. Based on the ranking analysis of high-frequency keywords, it can be observed that the popular research topics related to HMGB1 primarily concern molecular patterns and mechanisms of injury associated with HMGB1. Co-occurrence network maps also highlight several trending research directions from past studies, including cancer and cell death, neurological damage, cardiovascular disease, mechanisms of immune diseases, acute injury and oxidative stress, inflammatory molecules. The results incorporating time variation show that keywords are concentrated in areas such as diseases and their clinical diagnosis and treatment, cell death, mechanisms, and molecular patterns, among which neurological disorders have received significant attention in recent years. Critical literature and reference analysis reveals that the development trend of HMGB1-related research has become more diversified since around 2015. Meanwhile, new clusters have emerged: #1 (neuroinflammation), #0 (ferroptosis), #16 (metformin), and #18 (polycystic ovarian syndrome). These data reflect that these areas represent promising research directions in HMGB1-related studies.
HMGB1-associated cell death
HMGB1, which is typical of DAMPs and frequently leads to serious cell damage[44] . Necrosis, characterized by cell swelling and plasma membrane rupture, typically involves the passive release of HMGB1, which also plays a positive feedback role, promoting inflammation[45] . Consequently, HMGB1 is often used as a marker for necrosis[46] . In 2002, Scaffidi et al.'s pioneering study first elucidated HMGB1's role in necrotic cells, leading to increased attention on how HMGB1 is passively released from these cells to trigger sterile inflammation (Supplementary Table 6).
As shown in Figure 6A, HMGB1-related autophagy and apoptosis research is currently a hot field. The role of HMGB1-related autophagy in tumors is twofold. On the one hand, HMGB1 acts as a critical factor in cellular autophagy and mitosis[47,48] , which inhibits tumors. A deficiency of HMGB1 can trigger autophagy defects causing inflammation and genomic instability, leading to tumorigenesis[49,50] . On the other hand, autophagy mediated by HMGB1 enhances chemoresistance in cancer cells, such as colon, osteosarcoma, pancreatic, leukemia, gastric, and ovarian malignancies[51] . Numerous studies have demonstrated that inhibiting the expression of HMGB1 by RNAi increases the anticancer activity of drugs, and overexpression of HMGB1 by gene transfection increases drug resistance[50,52] , which may be related to the promotion of lactic acid production and glutamine metabolism[54] . It has been identified that HMGB1 acts as an anti-apoptotic protein within cells by regulating Bcl-2 family protein expression (transcription-dependent way), autophagy, and p53 localization (non-transcription-dependent way)[55,56] , in response to a variety of apoptotic stimuli including ultraviolet (UV) radiation, CD95, TRAIL, Casp-8, and Bax[57,58] . It is vital to note that HGBM1's ability to regulate apoptosis depends on its redox status[59] . Reducible HMGB1 inhibits apoptosis through binding to RAGE, but oxidized HMGB1 increases the drug's cytotoxicity and triggers apoptosis through the mitochondrial path[55] .
Keyword hotspot analysis further reveals that pyroptosis, ferroptosis, and immunogenic cell death represent cutting-edge research directions within the HMGB1 field (Figure 6, 7). Pyroptosis is a form of programmed cell death mediated by the Gasdermin family, characterized by membrane pore formation and cellular lysis, followed by the release of pro-inflammatory components such as HMGB1[60,61] . Pyroptosis can be mediated by caspase1, a core molecule of inflammatory vesicle activation[62] in which the inhibition of inflammatory vesicles reduces serum HMGB1 levels and protects against inflammatory damage in diabetic nephropathy[63] (70). Pyroptosis can be mediated by caspase1, a core molecule of inflammatory vesicle activation[62] in which the inhibition of inflammatory vesicles reduces serum HMGB1 levels and protects against inflammatory damage in diabetic nephropathy[63] (70). In ferroptosis, HMGB1 promotes iron accumulation through multiple mechanisms [64] and indirectly or directly increases iron death damage, such as by MAPK-dependent transferrin receptor (TFRC) expression[65] or acting as an autophagy regulator[66] . HMGB1 promotes ferroptosis through different mechanisms: by promoting MAPK-dependent TFRC expression, or acting as a regulator of autophagy that indirectly increases iron accumulation during ferroptosis injury. In addition, during ferroptosis, the inhibition of histone deacetylase (HDAC) facilitated the acetylation of HMGB1, thus promoting its release[67] . Immunogenic cell death is a type of cell death that occurs through the activation of adaptive immunity. This type of cell death occurs when tumor cells produce HMGB1, which then binds to TLR4 and RAGE[68] , thereby stimulating the body's immunological response to tumor cells, a mechanism often used in cancer treatment strategies[69,70] .
HMGB1-related diseases
Based on the results of the bibliometric analysis, as shown in Figure 6, research on HMGB1-related diseases initially focused primarily on critical care medicine, followed by cardiovascular diseases. During endotoxemia and sepsis, elevated levels of HMGB1 in tissues and circulation induce intestinal barrier dysfunction[71] and acute pulmonary injury[72] , and even fatal multi-organ failure[73] . In a manner analogous, HMGB1 acts as an intermediary of multiple organ failure during ischemia-reperfusion injury and is regarded as a biomarker of injury after liver and kidney transplantation[74,75] . HMGB1 levels are also associated with the urgency of several respiratory diseases, including asthma, acute respiratory distress syndrome, chronic obstructive pulmonary disease, and pneumonia[76] . Available data suggest that the release of HMGB1 is associated with the progression of SARS-CoV-2 from acute respiratory failure to sepsis[77] . In terms of cardiovascular disease, patients who suffer from atherosclerosis, myocardial infarction, cardiovascular inflammation, or heart failure and have elevated levels of HMGB1 in their serum are more likely to have a negative outcome from their disease[78,79] . Most studies imply that HMGB1 mediates injury through HMGB1-RAGE, with a particular focus on its role in atherosclerosis, where it is overexpressed in vascular smooth muscle cells, endothelial cells, foam cells, and macrophages[80,81] . Moreover, HMGB1 has been found to induce angiogenesis in tumor cells, involving the upregulation of neuropeptide 1, VEGF receptors 1 and 2, vascular endothelial growth factor A (VEGFA), platelet-derived growth factors, leukocyte adhesion molecules, and angiogenesis factors[82,83] . Similar literature clustering and topic evaluation of research fields indicate that as research progresses, the association of HMGB1 with cancer, autoimmune diseases, liver fibrosis, and diabetes is receiving increasing attention. Although HMGB1 is linked to a wide range of cancers[84,85] , such as hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), breast cancer, ovarian cancer, colorectal cancer, melanoma, esophageal cancer, mesothelioma, and others, it actually plays a dual role in the development of cancer[86] . On one hand, HMGB1 serves an anti-tumor role due to its contribution in the ICD process as well as the fact that it helps maintain the structure and integrity of the genome[87,88] . In contrast, a high level of HMGB1 production is linked to the development and spread of malignant tumors[84] . As a kind of autoimmune disease associated with HMGB1, rheumatoid arthritis is first reported, with elevated levels of HMGB1 in both the serum and the joint where inflammation occurred[89,90] . In one study, injection of HMGB1 into healthy joints resulted in NF-κB activation, IL-1β production, and arthritic symptoms in 80% of animals[91] . It may be hypothesized that HMGB1's complex formation with IL-1a, IL-1b, and LPS[92] is the pathogenic mechanism that causes the immunological and inflammatory response at the joints to be amplified. Serum HMGB1 levels are correlated with disease index and anti-dsDNA levels in SLE patients[93] . Notably, liver fibrosis, a common pathway of regression in chronic liver disease, also shows a high correlation with HMGB1, which may be related to its promotion of hepatic stellate cell proliferation and induction of fibronectin and collagen deposition[94,95] . In addition, HMGB1 has been found to promote the progression of both type 1 and type 2 diabetes mellitus[96,97] , which may be related to its mediated inflammatory and immune responses.
In recent years, research on HMGB1 in relation to neurological disorders has been at the forefront and has received considerable attention from a large number of scholars (Figure 8B). HMGB1 mediates neuroinflammation by binding to mediators such as RAGE and TLR4, and is associated with early brain injury (EBI) after traumatic brain injury (TBI) [98,99] and subarachnoid hemorrhage (SAH) [100] . In addition, HMGB1 is associated with various neurodegenerative illnesses such as Alzheimer's disease[101] , Huntington's disease[102] , Parkinson's disease[103] , and amyotrophic lateral sclerosis[104] . The results of keyword analysis showed that HMGB1 was closely related to the occurrence of epilepsy. It induces tissue damage and inflammatory responses through the TLR4-dependent pathway [105] and other complex receptor interactions such as IL-1R[106] , TLR2[107] , RAGE[108] , and NMDAR[109] , leading to seizures. At the same time, HMGB1, released from damaged neurons and astrocytes, may induce pain hypersensitivity by activating RAGE or TLR4 receptors, which may be an important factor in neuropathic pain [110,111] . After ischemic brain injury, HMGB1 is involved in the upregulation of hepcidin in astrocytes through ferroptosis and causes a sharp increase of cerebral iron levels[112] . In depression models, HMGB1 induces depressive-like behaviors by activating the TLR4/NF-κB signaling pathway in the hippocampus[113] , and sustained production of HMGB1/RAGE in microglia may increase susceptibility to depression[114] . However, the role and specific mechanisms of HMGB1 in these diseases still lack consistency and require further research to uncover its complex functions and potential therapeutic targets.
HMGB1-related clinical treatment
HMGB1 has become a focal point of research due to its detrimental roles in various diseases, particularly within the realm of critical care medicine. Highly cited literature analysis shows that therapeutic strategies for HMGB1 are rapidly developing, including anti-HMGB1 antibodies[115] , inhibitors[116] , insulin[117] , vasoactive intestinal peptides [118] and natural compounds such as quercetin [119] and Chinese herbal extracts [120] [121] . These methods have been shown to protect laboratory animals from endotoxemia or sepsis. Interestingly, HMGB1 antibodies attenuated septic injury dose-dependently[122] . In the Intensive Care Unit (ICU), HMGB1 serves as a biomarker for assessing the severity of sepsis through scoring systems like the Disseminated Intravascular Coagulation (DIC) score and Sequential Organ Failure Assessment (SOFA) score[123] . Furthermore, HMGB1 has been identified as a therapeutic target for acute-on-chronic liver failure (ACLF)[124] . In vivo studies have shown that treating mouse tumor models with anti-HMGB1 antibodies or inhibitors (e.g., BoxA) resulted in reduced tumor burden and improved survival rates[125] . Meanwhile, autophagy inhibitors like chloroquine (C.Q.) can slow down asbestos-induced mesothelioma carcinogenesis by inhibiting HMGB1-induced autophagy[126] . Treatment with TLR4 antagonist Eritoran reduced serum HMGB1 levels and attenuated hepatic ischemia-reperfusion injury[127] . For rheumatoid arthritis, oxaliplatin, glucocorticoids, and soluble RAGE, among others, have shown inhibitory effects on disease progression[90] . In severe cases of COVID-19, blocking the HMGB1-Ager pathway may help prevent the formation of ACE2[128] , and different HMGB1 gene polymorphisms and protein isoforms have been associated with varying disease progression and outcomes in patients[129] . In addition, inhibiting the TLR4 receptor with HMGB1 antibodies has been demonstrated to alleviate seizures in an erythropoietin-induced epilepsy model[130] .
In conclusion, basic medical research is currently working on the production of pharmaceuticals to treat related disorders by targeting the expression, release, or activation of HMGB1. In the future, firstly, additional clinical trials are required to elucidate the therapeutic impact of these treatments. Secondly, research in this field may concentrate on drugs that target the synthesis of HMGB1 but not limited to the expression, release, or activation. Thirdly, the biological effects of HMGB1 often vary due to different receptors and participating cells. Therefore, the development of drugs with higher specificity and effectiveness is warranted.
Limitations
The current work is to initially apply bibliometric visualization analysis to systematically review the research journey from the publication of the first article in the hgbm1-related field up to 2024. Nevertheless, this study has certain shortcomings unavoidably. First off, this study solely used data from the WOSCC database; it did not incorporate information from other databases like PubMed, the Cochrane Library, or Google Scholar. Despite WOSCC's thoroughness and dependability, the data in its database might contain some missing articles. Secondly, this study only included works in English, which could have skewed the findings. Thirdly, the data in this study could also be contradictory in other ways, such as if the same institution used different names at different times.
Conclusion
This study reviewed trends, hotspots, and frontiers of HMGB1-related research from 1976 to 2024 using bibliometric analysis. Influential journals in this area include International Immunopharmacology, Journal of Biological Chemistry, etc. In the field, reputable authors include Bianchi, M E, Tracey, Kevin J, Yang, H, and Andersson, U. The association between HMGB1 and neurological damage may be a route for future research. Immunology, inflammatory injury, cancer, and neurological damage are hot areas in the field. Our study elucidates the fundamental scientific knowledge and different interrelationships of HMGB1 and offers significant insights regarding current and emerging areas of HMGB1-related research. The results of this study should aid academics in better understanding current general trends, locating collaborators, and spotting more promising and innovative research topics.
Abbreviations
ACLF: acute-on-chronic liver failure; C.Q.: chloroquine; DAMP: damage-associated molecular pattern; DAMPs: damage-associated molecular patterns; DIC: Disseminated Intravascular Coagulation; EBI: early brain injury; HCC: hepatocellular carcinoma; HMG: high mobility group; HMGB1: High Mobility Group Protein B1; HDAC: histone deacetylase; IF: impact factor; ICU: Intensive Care Unit; JCR: Journal Citation Reports; LPS: lipopolysaccharide; LPS-mediated: Lipopolysaccharide-mediated; NSCLC: non-small cell lung cancer; PCA:principal component analysis; SOFA: Sequential Organ Failure Assessment; SAH: subarachnoid hemorrhage; TLR2 and TLR4: Toll-like receptors 2 and 4; TFRC: transferrin receptor; TBI: traumatic brain injury; UV: ultraviolet; VEGFA: vascular endothelial growth factor A; WOSCC: Web of Science Core Collection database
Supplementary Material
Supplementary methods, results, spectra, figures.
Acknowledgements
We sincerely thank Jianan Ding, Shuqian Zheng and Pinfei Pan for their suggestions and consultation on this article.
Author contributions
JW, LQ, GL, PD and YW: study conception, design and conduct, and full access to all the data in the study. LQ: draft the manuscript. YW: accept accountability for the correctness of the data analysis and interpretation. The manuscript was critically revised by JW, LQ, GL, PD and HL for key intellectual substance. The article's submission was reviewed and approved by all authors.
Funding information
Scientific Research Projects of Medical and Health Institutions of Longhua District, Shenzhen (No. 2021017), Medical Key Discipline of Longhua, Shenzhen (No. MKD202007090201), Project of Guangdong Medical Science and Technology Research Fund (No. A2022362), Shenzhen, Fundamental Research Project (No. JCYJ20220530165014033), College Students Innovation And Entrepreneurship Training Programme Project at Southern Medical University (grant no. 202112121203 to YW) and Special Funds for the Cultivation of Guangdong College Students' Scientific and Technological Innovation (grant no. pdjh2021a0095 to YW) all sponsored this study.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
[1] Goodwin, G. H., Sanders, C., & Johns, E. W. (1973). A new group of chromatin-associated proteins with a high content of acidic and basic amino acids [Journal Article]. Eur J Biochem, 38(1), 14-19. http://doi.org/10.1111/j.1432-1033.1973.tb03026.x
[2] Goodwin, G. H., & Johns, E. W. (1973). Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids [Journal Article]. Eur J Biochem, 40(1), 215-219. http://doi.org/10.1111/j.1432-1033.1973.tb03188.x
[3] Weir, H. M., Kraulis, P. J., Hill, C. S., Raine, A. R., Laue, E. D., & Thomas, J. O. (1993). Structure of the HMG box motif in the B-domain of HMG1 [Comparative Study; Journal Article; Research Support, Non-U.S. Gov't]. Embo Journal, 12(4), 1311-1319. http://doi.org/10.1002/j.1460-2075.1993.tb05776.x
[4] Balana, A. T., Mukherjee, A., Nagpal, H., Moon, S. P., Fierz, B., Vasquez, K. M., & Pratt, M. R. (2021). O-GlcNAcylation of High Mobility Group Box 1 (HMGB1) Alters Its DNA Binding and DNA Damage Processing Activities [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of the American Chemical Society, 143(39), 16030-16040. http://doi.org/10.1021/jacs.1c06192
[5] Polyanichko A, W. H. (2010). Structural organization of DNA-protein complexes of chromatin studied by vibrational and electronic circular dichroism. Spectroscopy-an International Journal, 3-4(24), 239-244.
[6] Song, M. J., Hwang, S., Wong, W., Round, J., Martinez-Guzman, D., Turpaz, Y., Liang, J., Wong, B., Johnson, R. C., Carey, M., & Sun, R. (2004). The DNA architectural protein HMGB1 facilitates RTA-mediated viral gene expression in gamma-2 herpesviruses [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. Journal of Virology, 78(23), 12940-12950. http://doi.org/10.1128/JVI.78.23.12940-12950.2004
[7] Ueda, T., Chou, H., Kawase, T., Shirakawa, H., & Yoshida, M. (2004). Acidic C-tail of HMGB1 is required for its target binding to nucleosome linker DNA and transcription stimulation [Journal Article; Research Support, Non-U.S. Gov't]. Biochemistry, 43(30), 9901-9908. http://doi.org/10.1021/bi035975l
[8] Wu, X. J., Chen, Y. Y., Guo, W. W., Li, T., Dong, H. B., Wang, W., Xie, M., Ma, G. L., & Pei, D. S. (2020). HMGB1 regulates SNAI1 during NSCLC metastasis, both directly, through transcriptional activation, and indirectly, in a RSF1-IT2-dependent manner [Journal Article; Research Support, Non-U.S. Gov't]. Molecular Oncology, 14(6), 1348-1364. http://doi.org/10.1002/1878-0261.12691
[9] Zhan, Z., Li, Q., Wu, P., Ye, Y., Tseng, H. Y., Zhang, L., & Zhang, X. D. (2012). Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1 [Journal Article; Research Support, Non-U.S. Gov't]. Autophagy, 8(1), 109-121. http://doi.org/10.4161/auto.8.1.18319
[10] Chen, R., Kang, R., & Tang, D. (2022). The mechanism of HMGB1 secretion and release [Journal Article; Research Support, Non-U.S. Gov't; Review]. Experimental and Molecular Medicine, 54(2), 91-102. http://doi.org/10.1038/s12276-022-00736-w
[11] Kim, Y. H., Kwak, M. S., Park, J. B., Lee, S. A., Choi, J. E., Cho, H. S., & Shin, J. S. (2016). N-linked glycosylation plays a crucial role in the secretion of HMGB1 [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cell Science, 129(1), 29-38. http://doi.org/10.1242/jcs.176412
[12] Lu, B., Wang, H., Andersson, U., & Tracey, K. J. (2013). Regulation of HMGB1 release by inflammasomes [Journal Article; Review]. Protein & Cell, 4(3), 163-167. http://doi.org/10.1007/s13238-012-2118-2
[13] Andersson, U., Tracey, K. J., & Yang, H. (2021). Post-Translational Modification of HMGB1 Disulfide Bonds in Stimulating and Inhibiting Inflammation [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cells, 10(12) http://doi.org/10.3390/cells10123323
[14] Watanabe, H., & Son, M. (2021). The Immune Tolerance Role of the HMGB1-RAGE Axis [Journal Article; Research Support, N.I.H., Extramural; Review]. Cells, 10(3) http://doi.org/10.3390/cells10030564
[15] Liu, X., Lu, B., Fu, J., Zhu, X., Song, E., & Song, Y. (2021). Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Hazardous Materials, 404(Pt B), 124050. http://doi.org/10.1016/j.jhazmat.2020.124050
[16] Mohamed, M. E., Kandeel, M., Abd, E. H., El-Beltagi, H. S., & Younis, N. S. (2022). The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFkappaB Pathway [Journal Article]. Antioxidants, 11(3) http://doi.org/10.3390/antiox11030535
[17] Denning, N. L., Aziz, M., Gurien, S. D., & Wang, P. (2019). DAMPs and NETs in Sepsis [Journal Article; Research Support, N.I.H., Extramural; Review]. Frontiers in Immunology, 10, 2536. http://doi.org/10.3389/fimmu.2019.02536
[18] Rani, M., Nicholson, S. E., Zhang, Q., & Schwacha, M. G. (2017). Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation [Journal Article]. Burns, 43(2), 297-303. http://doi.org/10.1016/j.burns.2016.10.001
[19] Lee, S. A., Kwak, M. S., Kim, S., & Shin, J. S. (2014). The role of high mobility group box 1 in innate immunity [Journal Article; Research Support, Non-U.S. Gov't; Review]. Yonsei Medical Journal, 55(5), 1165-1176. http://doi.org/10.3349/ymj.2014.55.5.1165
[20] Xu, K., Ren, X., Ju, B., Aihaiti, Y., Cai, Y., Zhang, Y., He, L., & Wang, J. (2020). Clinical markers combined with HMGB1 polymorphisms to predict efficacy of conventional DMARDs in rheumatoid arthritis patients [Journal Article; Research Support, Non-U.S. Gov't]. Clinical Immunology, 221, 108592. http://doi.org/10.1016/j.clim.2020.108592
[21] Wu, R., Wang, N., Comish, P. B., Tang, D., & Kang, R. (2021). Inflammasome-Dependent Coagulation Activation in Sepsis [Journal Article; Review]. Frontiers in Immunology, 12, 641750. http://doi.org/10.3389/fimmu.2021.641750
[22] Foglio, E., Pellegrini, L., Russo, M. A., & Limana, F. (2022). HMGB1-Mediated Activation of the Inflammatory-Reparative Response Following Myocardial Infarction [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cells, 11(2) http://doi.org/10.3390/cells11020216
[23] Meng, X., Su, W., Tao, X., Sun, M., Ying, R., Wei, W., & Wang, B. (2018). Oxidation Prevents HMGB1 Inhibition on PDGF-Induced Differentiation of Multipotent Vascular Stem Cells to Smooth Muscle Cells: A Possible Mechanism Linking Oxidative Stress to Atherosclerosis [Journal Article]. Biomed Research International, 2018, 4019814. http://doi.org/10.1155/2018/4019814
[24] Hemmer, S., Senger, S., Griessenauer, C. J., Simgen, A., Oertel, J., Geisel, J., & Hendrix, P. (2022). Admission serum high mobility group box 1 (HMGB1) protein predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage [Journal Article; Observational Study]. Neurosurgical Review, 45(1), 807-817. http://doi.org/10.1007/s10143-021-01607-0
[25] Yang, L., Zhou, L., Wang, X., Wang, W., & Wang, J. (2020). Inhibition of HMGB1 involved in the protective of salidroside on liver injury in diabetes mice [Journal Article]. International Immunopharmacology, 89(Pt A), 106987. http://doi.org/10.1016/j.intimp.2020.106987
[26] Tanaka, A., Ito, T., Kibata, K., Inagaki-Katashiba, N., Amuro, H., Nishizawa, T., Son, Y., Ozaki, Y., & Nomura, S. (2019). Serum high-mobility group box 1 is correlated with interferon-alpha and may predict disease activity in patients with systemic lupus erythematosus [Journal Article]. Lupus, 28(9), 1120-1127. http://doi.org/10.1177/0961203319862865
[27] Ho, W. I., Hu, Y., Cheng, C. W., Wei, R., Yang, J., Li, N., Au, K. W., Tse, Y. L., Wang, Q., Ng, K. M., Esteban, M. A., & Tse, H. F. (2022). Liposome-encapsulated curcumin attenuates HMGB1-mediated hepatic inflammation and fibrosis in a murine model of Wilson's disease [Journal Article]. Biomedicine & Pharmacotherapy, 152, 113197. http://doi.org/10.1016/j.biopha.2022.113197
[28] Sui, H., Luo, M., Miao, Y., Cheng, W., Wen, S., Zhao, B., Li, Y., Qiao, Z., Liu, Y., & Xu, C. (2020). Cystic fibrosis transmembrane conductance regulator ameliorates lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/AKT/mTOR pathway in mice [Journal Article; Research Support, Non-U.S. Gov't]. Respiratory Physiology & Neurobiology, 273, 103338. http://doi.org/10.1016/j.resp.2019.103338
[29] Jankauskaite, L., Malinauskas, M., & Mickeviciute, G. C. (2022). HMGB1: A Potential Target of Nervus Vagus Stimulation in Pediatric SARS-CoV-2-Induced ALI/ARDS [Journal Article]. Frontiers in Pediatrics, 10, 884539. http://doi.org/10.3389/fped.2022.884539
[30] Teo, H. S. A., Schlichtner, S., Yasinska, I. M., Sakhnevych, S. S., Fiedler, W., Wellbrock, J., Berger, S. M., Klenova, E., Gibbs, B. F., Fasler-Kan, E., & Sumbayev, V. V. (2021). High Mobility Group Box 1 (HMGB1) Induces Toll-Like Receptor 4-Mediated Production of the Immunosuppressive Protein Galectin-9 in Human Cancer Cells [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 12, 675731. http://doi.org/10.3389/fimmu.2021.675731
[31] Simon, D. W., Aneja, R. K., Alexander, H., Bell, M. J., Bayir, H., Kochanek, P. M., & Clark, R. (2018). Minocycline Attenuates High Mobility Group Box 1 Translocation, Microglial Activation, and Thalamic Neurodegeneration after Traumatic Brain Injury in Post-Natal Day 17 Rats [Journal Article; Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 35(1), 130-138. http://doi.org/10.1089/neu.2017.5093
[32] Hicks, D., Wouters, P., Waltman, L., de Rijcke, S., & Rafols, I. (2015). Bibliometrics: The Leiden Manifesto for research metrics [Historical Article; Journal Article]. Nature, 520(7548), 429-431. http://doi.org/10.1038/520429a
[33] Deng, P., Shi, H., Pan, X., Liang, H., Wang, S., Wu, J., Zhang, W., Huang, F., Sun, X., Zhu, H., & Chen, Z. (2022). Worldwide Research Trends on Diabetic Foot Ulcers (2004-2020): Suggestions for Researchers [Journal Article]. Journal of Diabetes Research, 2022, 7991031. http://doi.org/10.1155/2022/7991031
[34] Ninkov, A., Frank, J. R., & Maggio, L. A. (2022). Bibliometrics: Methods for studying academic publishing [Journal Article]. Perspectives On Medical Education, 11(3), 173-176. http://doi.org/10.1007/s40037-021-00695-4
[35] Shi, X., Wang, S., Wu, Y., Li, Q., Zhang, T., Min, K., Feng, D., Liu, M., Wei, J., Zhu, L., Mo, W., Xiao, Z., Yang, H., Chen, Y., & Lv, X. (2022). A Bibliometric Analysis of the Innate Immune DNA Sensing cGAS-STING Pathway from 2013 to 2021 [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 13, 916383. http://doi.org/10.3389/fimmu.2022.916383
[36] Ma, D., Yang, B., Guan, B., Song, L., Liu, Q., Fan, Y., Zhao, L., Wang, T., Zhang, Z., Gao, Z., Li, S., & Xu, H. (2021). A Bibliometric Analysis of Pyroptosis From 2001 to 2021 [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 12, 731933. http://doi.org/10.3389/fimmu.2021.731933
[37] Dong, X., Tan, Y., Zhuang, D., Hu, T., & Zhao, M. (2022). Global Characteristics and Trends in Research on Ferroptosis: A Data-Driven Bibliometric Study [Journal Article; Review]. Oxidative Medicine and Cellular Longevity, 2022, 8661864. http://doi.org/10.1155/2022/8661864
[38] Chen, C. (2006). CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. Journal of the American Society for Information Science and Technology, 57(3), 359-377. http://doi.org/10.1002/asi.20317
[39] van Eck, N. J., & Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping [Journal Article]. Scientometrics, 84(2), 523-538. http://doi.org/10.1007/s11192-009-0146-3
[40] Aria, M., & Cuccurullo, C. (2018). bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. Journal of Informetrics, 11(4), 959-975.
[41] Scaffidi, P., Misteli, T., & Bianchi, M. E. (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation [Journal Article; Research Support, Non-U.S. Gov't]. Nature, 418(6894), 191-195. http://doi.org/10.1038/nature00858
[42] Park, J. S., Svetkauskaite, D., He, Q., Kim, J. Y., Strassheim, D., Ishizaka, A., & Abraham, E. (2004). Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein [Journal Article; Research Support, U.S. Gov't, P.H.S.]. Journal of Biological Chemistry, 279(9), 7370-7377. http://doi.org/10.1074/jbc.M306793200
[43] Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., Huang, J., Yu, Y., Fan, X. G., Yan, Z., Sun, X., Wang, H., Wang, Q., Tsung, A., Billiar, T. R., Zeh, H. R., Lotze, M. T., & Tang, D. (2014). HMGB1 in health and disease [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Molecular Aspects of Medicine, 40, 1-116. http://doi.org/10.1016/j.mam.2014.05.001
[44] Chen, R., Kang, R., & Tang, D. (2022). The mechanism of HMGB1 secretion and release [Journal Article; Research Support, Non-U.S. Gov't; Review]. Experimental and Molecular Medicine, 54(2), 91-102. http://doi.org/10.1038/s12276-022-00736-w
[45] Lee, S. Y., Ju, M. K., Jeon, H. M., Jeong, E. K., Lee, Y. J., Kim, C. H., Park, H. G., Han, S. I., & Kang, H. S. (2018). Regulation of Tumor Progression by Programmed Necrosis [Journal Article; Review]. Oxidative Medicine and Cellular Longevity, 2018, 3537471. http://doi.org/10.1155/2018/3537471
[46] Stelmasiak, M., Mikaszewska-Sokolewicz, M., NiewiNski, G., BaLan, B. J., & SLotwiNski, R. (2020). The soluble tumor necrosis factor receptor 1 as a potential early diagnostic and prognostic markers in intensive care unit patients with severe infections [Journal Article]. Central European Journal of Immunology, 45(2), 160-169. http://doi.org/10.5114/ceji.2020.97903
[47] Tang, D., Kang, R., Livesey, K. M., Cheh, C. W., Farkas, A., Loughran, P., Hoppe, G., Bianchi, M. E., Tracey, K. J., Zeh, H. R., & Lotze, M. T. (2010). Endogenous HMGB1 regulates autophagy [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of Cell Biology, 190(5), 881-892. http://doi.org/10.1083/jcb.200911078
[48] Tang, D., Kang, R., Livesey, K. M., Kroemer, G., Billiar, T. R., Van Houten, B., Zeh, H. R., & Lotze, M. T. (2011). High-mobility group box 1 is essential for mitochondrial quality control [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cell Metabolism, 13(6), 701-711. http://doi.org/10.1016/j.cmet.2011.04.008
[49] Kang, R., Zhang, Q., Zeh, H. R., Lotze, M. T., & Tang, D. (2013). HMGB1 in cancer: good, bad, or both? [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Clinical Cancer Research, 19(15), 4046-4057. http://doi.org/10.1158/1078-0432.CCR-13-0495
[50] Livesey, K. M., Kang, R., Vernon, P., Buchser, W., Loughran, P., Watkins, S. C., Zhang, L., Manfredi, J. J., Zeh, H. R., Li, L., Lotze, M. T., & Tang, D. (2012). p53/HMGB1 complexes regulate autophagy and apoptosis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cancer Research, 72(8), 1996-2005. http://doi.org/10.1158/0008-5472.CAN-11-2291
[51] Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., Huang, J., Yu, Y., Fan, X. G., Yan, Z., Sun, X., Wang, H., Wang, Q., Tsung, A., Billiar, T. R., Zeh, H. R., Lotze, M. T., & Tang, D. (2014). HMGB1 in health and disease [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Molecular Aspects of Medicine, 40, 1-116. http://doi.org/10.1016/j.mam.2014.05.001
[52] Huang, J., Ni, J., Liu, K., Yu, Y., Xie, M., Kang, R., Vernon, P., Cao, L., & Tang, D. (2012). HMGB1 promotes drug resistance in osteosarcoma [Journal Article; Research Support, Non-U.S. Gov't]. Cancer Research, 72(1), 230-238. http://doi.org/10.1158/0008-5472.CAN-11-2001
[53] Liu, L., Yang, M., Kang, R., Wang, Z., Zhao, Y., Yu, Y., Xie, M., Yin, X., Livesey, K. M., Lotze, M. T., Tang, D., & Cao, L. (2011). HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells [Journal Article; Research Support, Non-U.S. Gov't]. Leukemia, 25(1), 23-31. http://doi.org/10.1038/leu.2010.225
[54] Luo, Y., Yoneda, J., Ohmori, H., Sasaki, T., Shimbo, K., Eto, S., Kato, Y., Miyano, H., Kobayashi, T., Sasahira, T., Chihara, Y., & Kuniyasu, H. (2014). Cancer usurps skeletal muscle as an energy repository [Journal Article; Research Support, Non-U.S. Gov't]. Cancer Research, 74(1), 330-340. http://doi.org/10.1158/0008-5472.CAN-13-1052
[55] Kang, R., Zeh, H. J., Lotze, M. T., & Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Cell Death and Differentiation, 18(4), 571-580. http://doi.org/10.1038/cdd.2010.191
[56] Liu, B., Gan, X., Zhao, Y., Gao, J., & Yu, H. (2021). Inhibition of HMGB1 reduced high glucose-induced BMSCs apoptosis via activation of AMPK and regulation of mitochondrial functions [Journal Article]. Journal of Physiology and Biochemistry, 77(2), 227-235. http://doi.org/10.1007/s13105-021-00784-2
[57] Brezniceanu, M. L., Volp, K., Bosser, S., Solbach, C., Lichter, P., Joos, S., & Zornig, M. (2003). HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma [Journal Article]. Faseb Journal, 17(10), 1295-1297. http://doi.org/10.1096/fj.02-0621fje
[58] Jiang, H., Hu, X., Zhang, H., & Li, W. (2017). Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression [Journal Article]. Radiation Oncology, 12(1), 65. http://doi.org/10.1186/s13014-017-0802-3
[59] Zhi, S. M., Fang, G. X., Xie, X. M., Liu, L. H., Yan, J., Liu, D. B., & Yu, H. Y. (2020). Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling [Journal Article; Research Support, Non-U.S. Gov't]. European Review for Medical and Pharmacological Sciences, 24(3), 1524-1536. http://doi.org/10.26355/eurrev_202002_20211
[60] Huo, J., Shen, Y., Zhang, Y., & Shen, L. (2022). BI 2536 induces gasdermin E-dependent pyroptosis in ovarian cancer [Journal Article]. Frontiers in Oncology, 12, 963928. http://doi.org/10.3389/fonc.2022.963928
[61] Zeng, C. Y., Li, C. G., Shu, J. X., Xu, L. H., Ouyang, D. Y., Mai, F. Y., Zeng, Q. Z., Zhang, C. C., Li, R. M., & He, X. H. (2019). ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages [Journal Article; Research Support, Non-U.S. Gov't]. Apoptosis, 24(9-10), 703-717. http://doi.org/10.1007/s10495-019-01551-x
[62] Kamo N, K. B. G. A. (2013). ASC/Caspase-1/IL-1 beta Signaling Triggers Inflammatory Responses by Promoting HMGB1 Induction in Liver Ischemia/Reperfusion Injury. Hepatology, 1(58), 351-362.
[63] Li, Y., Yuan, Y., Huang, Z. X., Chen, H., Lan, R., Wang, Z., Lai, K., Chen, H., Chen, Z., Zou, Z., Ma, H. B., Lan, H. Y., Mak, T. W., & Xu, Y. (2021). GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy [Journal Article; Research Support, Non-U.S. Gov't]. Cell Death and Differentiation, 28(8), 2333-2350. http://doi.org/10.1038/s41418-021-00755-6
[64] Chen, X., Yu, C., Kang, R., Kroemer, G., & Tang, D. (2021). Cellular degradation systems in ferroptosis [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cell Death and Differentiation, 28(4), 1135-1148. http://doi.org/10.1038/s41418-020-00728-1
[65] Lin, C., Hu, R., Sun, F., & Liang, W. (2022). Ferroptosis-based molecular prognostic model for adrenocortical carcinoma based on least absolute shrinkage and selection operator regression [Journal Article]. Journal of Clinical Laboratory Analysis, 36(6), e24465. http://doi.org/10.1002/jcla.24465
[66] Hu, N., Bai, L., Dai, E., Han, L., Kang, R., Li, H., & Tang, D. (2021). Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis [Journal Article]. Biochemical and Biophysical Research Communications, 536, 100-106. http://doi.org/10.1016/j.bbrc.2020.12.066
[67] Wen, Q., Liu, J., Kang, R., Zhou, B., & Tang, D. (2019). The release and activity of HMGB1 in ferroptosis [Journal Article; Research Support, Non-U.S. Gov't]. Biochemical and Biophysical Research Communications, 510(2), 278-283. http://doi.org/10.1016/j.bbrc.2019.01.090
[68] He, C., Sun, S., Zhang, Y., Xie, F., & Li, S. (2021). The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer [Journal Article; Research Support, Non-U.S. Gov't]. OncoImmunology, 10(1), 1897295. http://doi.org/10.1080/2162402X.2021.1897295
[69] Cebrian, M. J., Bauden, M., Andersson, R., Holdenrieder, S., & Ansari, D. (2016). Paradoxical Role of HMGB1 in Pancreatic Cancer: Tumor Suppressor or Tumor Promoter? [Journal Article; Review]. Anticancer Research, 36(9), 4381-4389. http://doi.org/10.21873/anticanres.10981
[70] Dong, H., Zhang, L., & Liu, S. (2022). Targeting HMGB1: An available Therapeutic Strategy for Breast Cancer Therapy [Journal Article; Research Support, Non-U.S. Gov't; Review]. International Journal of Biological Sciences, 18(8), 3421-3434. http://doi.org/10.7150/ijbs.73504
[71] Wang, H., Zhang, S., Zhao, H., Qin, H., Zhang, J., Dong, J., Zhang, H., Liu, X., Zhao, Z., Zhao, Y., Shao, M., Wu, F., & Zhang, W. (2020). Carbon Monoxide Inhibits the Expression of Proteins Associated with Intestinal Mucosal Pyroptosis in a Rat Model of Sepsis Induced by Cecal Ligation and Puncture [Journal Article]. Medical Science Monitor, 26, e920668. http://doi.org/10.12659/MSM.920668
[72] Chen, G., Hou, Y., Li, X., Pan, R., & Zhao, D. (2021). Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-kappaB pathway and NLRP3 inflammasome [Journal Article]. International Immunopharmacology, 96, 107623. http://doi.org/10.1016/j.intimp.2021.107623
[73] Li, Y., Zhu, H., Pan, L., Zhang, B., & Che, H. (2021). microRNA-103a-3p confers protection against lipopolysaccharide-induced sepsis and consequent multiple organ dysfunction syndrome by targeting HMGB1 [Journal Article]. Infection Genetics and Evolution, 89, 104681. http://doi.org/10.1016/j.meegid.2020.104681
[74] Kruger, B., Krick, S., Dhillon, N., Lerner, S. M., Ames, S., Bromberg, J. S., Lin, M., Walsh, L., Vella, J., Fischereder, M., Kramer, B. K., Colvin, R. B., Heeger, P. S., Murphy, B. T., & Schroppel, B. (2009). Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3390-3395. http://doi.org/10.1073/pnas.0810169106
[75] Panisello-Rosello, A., Verde, E., Lopez, A., Flores, M., Folch-Puy, E., Rolo, A., Palmeira, C., Hotter, G., Carbonell, T., Adam, R., & Rosello-Catafau, J. (2018). Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK [Journal Article]. International Journal of Molecular Sciences, 19(2) http://doi.org/10.3390/ijms19020348
[76] Manti, S., Harford, T. J., Salpietro, C., Rezaee, F., & Piedimonte, G. (2018). Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus [Journal Article; Research Support, N.I.H., Extramural]. Pediatric Research, 83(5), 1049-1056. http://doi.org/10.1038/pr.2018.6
[77] Wyganowska-Swiatkowska, M., Nohawica, M., Grocholewicz, K., & Nowak, G. (2020). Influence of Herbal Medicines on HMGB1 Release, SARS-CoV-2 Viral Attachment, Acute Respiratory Failure, and Sepsis. A Literature Review [Journal Article; Review]. International Journal of Molecular Sciences, 21(13) http://doi.org/10.3390/ijms21134639
[78] Ogura, Y., Fukuchi, K., Morimoto, H., Yuki, T., Otsuka, M., Shimauchi, T., Honda, T., & Tokura, Y. (2022). Elevation of circulating neutrophil extracellular traps, interleukin (IL)-8, IL-22, and vascular endothelial growth factor in patients with venomous snake mamushi (Gloydius blomhoffii) bites [Journal Article]. Journal of Dermatology, 49(1), 124-132. http://doi.org/10.1111/1346-8138.16181
[79] Wahid, A., Chen, W., Wang, X., & Tang, X. (2021). High-mobility group box 1 serves as an inflammation driver of cardiovascular disease [Journal Article; Review]. Biomedicine & Pharmacotherapy, 139, 111555. http://doi.org/10.1016/j.biopha.2021.111555
[80] Kalinina, N., Agrotis, A., Antropova, Y., DiVitto, G., Kanellakis, P., Kostolias, G., Ilyinskaya, O., Tararak, E., & Bobik, A. (2004). Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines [Journal Article]. Arteriosclerosis Thrombosis and Vascular Biology, 24(12), 2320-2325. http://doi.org/10.1161/01.ATV.0000145573.36113.8a
[81] Liu, M., Yu, Y., Jiang, H., Zhang, L., Zhang, P. P., Yu, P., Jia, J. G., Chen, R. Z., Zou, Y. Z., & Ge, J. B. (2013). Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis [Journal Article; Research Support, Non-U.S. Gov't]. Acta Pharmacologica Sinica, 34(6), 830-836. http://doi.org/10.1038/aps.2013.8
[82] Feng, Y., Ke, J., Cao, P., Deng, M., Li, J., Cai, H., Meng, Q., Li, Y., & Long, X. (2018). HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cellular and Molecular Medicine, 22(2), 1283-1291. http://doi.org/10.1111/jcmm.13410
[83] van Beijnum, J. R., Nowak-Sliwinska, P., van den Boezem, E., Hautvast, P., Buurman, W. A., & Griffioen, A. W. (2013). Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. Oncogene, 32(3), 363-374. http://doi.org/10.1038/onc.2012.49
[84] Rapoport, B. L., Steel, H. C., Theron, A. J., Heyman, L., Smit, T., Ramdas, Y., & Anderson, R. (2020). High Mobility Group Box 1 in Human Cancer [Journal Article; Review]. Cells, 9(7) http://doi.org/10.3390/cells9071664
[85] Wang, J. D., Wang, Y. Y., Lin, S. Y., Chang, C. Y., Li, J. R., Huang, S. W., Chen, W. Y., Liao, S. L., & Chen, C. J. (2021). Exosomal HMGB1 Promoted Cancer Malignancy [Journal Article]. Cancers, 13(4) http://doi.org/10.3390/cancers13040877
[86] He, S. J., Cheng, J., Feng, X., Yu, Y., Tian, L., & Huang, Q. (2017). The dual role and therapeutic potential of high-mobility group box 1 in cancer [Journal Article; Review]. Oncotarget, 8(38), 64534-64550. http://doi.org/10.18632/oncotarget.17885
[87] Xu, T., Jiang, L., & Wang, Z. (2019). The progression of HMGB1-induced autophagy in cancer biology [Journal Article; Review]. OncoTargets and Therapy, 12, 365-377. http://doi.org/10.2147/OTT.S185876
[88] Mandke, P., & Vasquez, K. M. (2019). Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses [Journal Article; Research Support, N.I.H., Extramural; Review]. Dna Repair, 83, 102701. http://doi.org/10.1016/j.dnarep.2019.102701
[89] Goldstein, R. S., Bruchfeld, A., Yang, L., Qureshi, A. R., Gallowitsch-Puerta, M., Patel, N. B., Huston, B. J., Chavan, S., Rosas-Ballina, M., Gregersen, P. K., Czura, C. J., Sloan, R. P., Sama, A. E., & Tracey, K. J. (2007). Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis [Comparative Study; Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Molecular Medicine, 13(3-4), 210-215. http://doi.org/10.2119/2006-00108.Goldstein
[90] Kaur, I., Behl, T., Bungau, S., Kumar, A., Mehta, V., Setia, D., Uddin, M. S., Zengin, G., Aleya, L., & Arora, S. (2020). Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis [Journal Article; Review]. Life Sciences, 258, 118164. http://doi.org/10.1016/j.lfs.2020.118164
[91] Garcia-Arnandis, I., Guillen, M. I., Gomar, F., Pelletier, J. P., Martel-Pelletier, J., & Alcaraz, M. J. (2010). High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes [Journal Article; Research Support, Non-U.S. Gov't]. Arthritis Research & Therapy, 12(4), R165. http://doi.org/10.1186/ar3124
[92] Qin, Y., Chen, Y., Wang, W., Wang, Z., Tang, G., Zhang, P., He, Z., Liu, Y., Dai, S. M., & Shen, Q. (2014). HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype [Journal Article; Research Support, Non-U.S. Gov't]. Cell Death & Disease, 5(2), e1077. http://doi.org/10.1038/cddis.2014.48
[93] Li, R., Jia, F., Ren, K., Luo, M., Min, X., Xiao, S., & Xia, Y. (2021). Fibroblast growth factor inducible 14 signaling facilitates anti-dsDNA IgG penetration into mesangial cells [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cellular Physiology, 236(1), 249-259. http://doi.org/10.1002/jcp.29838
[94] Kuwano, A., Kohjima, M., Suzuki, H., Yamasaki, A., Ohashi, T., Imoto, K., Kurokawa, M., Morita, Y., Kato, M., & Ogawa, Y. (2019). Recombinant human soluble thrombomodulin ameliorates acetaminophen-induced liver toxicity in mice [Journal Article]. Experimental and Therapeutic Medicine, 18(2), 1323-1330. http://doi.org/10.3892/etm.2019.7665
[95] Regan, J. K., Kannan, P. S., Kemp, M. W., Kramer, B. W., Newnham, J. P., Jobe, A. H., & Kallapur, S. G. (2016). Damage-Associated Molecular Pattern and Fetal Membrane Vascular Injury and Collagen Disorganization in Lipopolysaccharide-Induced Intra-amniotic Inflammation in Fetal Sheep [Journal Article; Research Support, N.I.H., Extramural]. Reproductive Sciences, 23(1), 69-80. http://doi.org/10.1177/1933719115594014
[96] Chen, Y., Qiao, F., Zhao, Y., Wang, Y., & Liu, G. (2015). HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose [Journal Article; Research Support, Non-U.S. Gov't]. International Journal of Clinical and Experimental Pathology, 8(6), 6683-6691.
[97] Wang, X., Feng, C., Qiao, Y., & Zhao, X. (2018). Sigma 1 receptor mediated HMGB1 expression in spinal cord is involved in the development of diabetic neuropathic pain [Journal Article; Research Support, Non-U.S. Gov't]. Neuroscience Letters, 668, 164-168. http://doi.org/10.1016/j.neulet.2018.02.002
[98] Gao, T. L., Yuan, X. T., Yang, D., Dai, H. L., Wang, W. J., Peng, X., Shao, H. J., Jin, Z. F., & Fu, Z. J. (2012). Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury [Comparative Study; Journal Article; Research Support, Non-U.S. Gov't]. Journal of Trauma and Acute Care Surgery, 72(3), 643-649. http://doi.org/10.1097/TA.0b013e31823c54a6
[99] Okuma, Y., Liu, K., Wake, H., Zhang, J., Maruo, T., Date, I., Yoshino, T., Ohtsuka, A., Otani, N., Tomura, S., Shima, K., Yamamoto, Y., Yamamoto, H., Takahashi, H. K., Mori, S., & Nishibori, M. (2012). Anti-high mobility group box-1 antibody therapy for traumatic brain injury [Journal Article; Research Support, Non-U.S. Gov't]. Annals of Neurology, 72(3), 373-384. http://doi.org/10.1002/ana.23602
[100] Haruma, J., Teshigawara, K., Hishikawa, T., Wang, D., Liu, K., Wake, H., Mori, S., Takahashi, H. K., Sugiu, K., Date, I., & Nishibori, M. (2016). Anti-high mobility group box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats [Journal Article; Research Support, Non-U.S. Gov't]. Scientific Reports, 6, 37755. http://doi.org/10.1038/srep37755
[101] Fujita, K., Motoki, K., Tagawa, K., Chen, X., Hama, H., Nakajima, K., Homma, H., Tamura, T., Watanabe, H., Katsuno, M., Matsumi, C., Kajikawa, M., Saito, T., Saido, T., Sobue, G., Miyawaki, A., & Okazawa, H. (2016). HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease [Journal Article; Research Support, Non-U.S. Gov't]. Scientific Reports, 6, 31895. http://doi.org/10.1038/srep31895
[102] Angelopoulou, E., Paudel, Y. N., & Piperi, C. (2020). Exploring the role of high-mobility group box 1 (HMGB1) protein in the pathogenesis of Huntington's disease [Journal Article; Research Support, Non-U.S. Gov't; Review]. Journal of Molecular Medicine-Jmm, 98(3), 325-334. http://doi.org/10.1007/s00109-020-01885-z
[103] Ren, Q., Jiang, X., Paudel, Y. N., Gao, X., Gao, D., Zhang, P., Sheng, W., Shang, X., Liu, K., Zhang, X., & Jin, M. (2022). Co-treatment with natural HMGB1 inhibitor Glycyrrhizin exerts neuroprotection and reverses Parkinson's disease like pathology in Zebrafish [Journal Article]. Journal of Ethnopharmacology, 292, 115234. http://doi.org/10.1016/j.jep.2022.115234
[104] Paudel, Y. N., Angelopoulou, E., Piperi, C., Othman, I., & Shaikh, M. F. (2020). Implication of HMGB1 signaling pathways in Amyotrophic lateral sclerosis (ALS): From molecular mechanisms to pre-clinical results [Journal Article; Research Support, Non-U.S. Gov't; Review]. Pharmacological Research, 156, 104792. http://doi.org/10.1016/j.phrs.2020.104792
[105] Kleen, J. K., & Holmes, G. L. (2010). Taming TLR4 may ease seizures [Comment; News]. Nature Medicine, 16(4), 369-370. http://doi.org/10.1038/nm0410-369
[106] Maroso, M., Balosso, S., Ravizza, T., Liu, J., Bianchi, M. E., & Vezzani, A. (2011). Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Internal Medicine, 270(4), 319-326. http://doi.org/10.1111/j.1365-2796.2011.02431.x
[107] Vezzani, A., Maroso, M., Balosso, S., Sanchez, M. A., & Bartfai, T. (2011). IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures [Journal Article; Review]. Brain Behavior and Immunity, 25(7), 1281-1289. http://doi.org/10.1016/j.bbi.2011.03.018
[108] Zurolo, E., Iyer, A., Maroso, M., Carbonell, C., Anink, J. J., Ravizza, T., Fluiter, K., Spliet, W. G., van Rijen, P. C., Vezzani, A., & Aronica, E. (2011). Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development [Journal Article; Research Support, Non-U.S. Gov't]. Brain, 134(Pt 4), 1015-1032. http://doi.org/10.1093/brain/awr032
[109] Balosso, S., Liu, J., Bianchi, M. E., & Vezzani, A. (2014). Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons [Journal Article; Research Support, Non-U.S. Gov't]. Antioxidants & Redox Signaling, 21(12), 1726-1740. http://doi.org/10.1089/ars.2013.5349
[110] Feldman, P., Due, M. R., Ripsch, M. S., Khanna, R., & White, F. A. (2012). The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. Journal of Neuroinflammation, 9, 180. http://doi.org/10.1186/1742-2094-9-180
[111] Kuang, X., Huang, Y., Gu, H. F., Zu, X. Y., Zou, W. Y., Song, Z. B., & Guo, Q. L. (2012). Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats [Journal Article]. European Journal of Pharmacology, 676(1-3), 51-56. http://doi.org/10.1016/j.ejphar.2011.11.037
[112] Davaanyam, D., Lee, H., Seol, S. I., Oh, S. A., Kim, S. W., & Lee, J. K. (2023). HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain [Journal Article; Research Support, Non-U.S. Gov't]. Experimental and Molecular Medicine, 55(11), 2402-2416. http://doi.org/10.1038/s12276-023-01111-z
[113] Liu, L., Dong, Y., Shan, X., Li, L., Xia, B., & Wang, H. (2019). Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo [Journal Article]. Molecules, 24(2) http://doi.org/10.3390/molecules24020326
[114] Franklin, T. C., Wohleb, E. S., Zhang, Y., Fogaca, M., Hare, B., & Duman, R. S. (2018). Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior [Journal Article]. Biological Psychiatry, 83(1), 50-60. http://doi.org/10.1016/j.biopsych.2017.06.034
[115] Valdes-Ferrer, S. I., Rosas-Ballina, M., Olofsson, P. S., Lu, B., Dancho, M. E., Ochani, M., Li, J. H., Scheinerman, J. A., Katz, D. A., Levine, Y. A., Hudson, L. K., Yang, H., Pavlov, V. A., Roth, J., Blanc, L., Antoine, D. J., Chavan, S. S., Andersson, U., Diamond, B., & Tracey, K. J. (2013). HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of Internal Medicine, 274(4), 381-390. http://doi.org/10.1111/joim.12104
[116] Zhu, C., Chen, T., & Liu, B. (2018). Inhibitory effects of miR-25 targeting HMGB1 on macrophage secretion of inflammatory cytokines in sepsis [Journal Article]. Oncology Letters, 16(4), 5027-5033. http://doi.org/10.3892/ol.2018.9308
[117] Hagiwara, S., Iwasaka, H., Hasegawa, A., Koga, H., & Noguchi, T. (2008). Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model [Journal Article]. Critical Care Medicine, 36(8), 2407-2413. http://doi.org/10.1097/CCM.0b013e318180b3ba
[118] Chorny, A., & Delgado, M. (2008). Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. American Journal of Pathology, 172(5), 1297-1307. http://doi.org/10.2353/ajpath.2008.070969
[119] Yang, M., Cao, L., Xie, M., Yu, Y., Kang, R., Yang, L., Zhao, M., & Tang, D. (2013). Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Biochemical Pharmacology, 86(3), 410-418. http://doi.org/10.1016/j.bcp.2013.05.013
[120] Wang, B., Koga, K., Osuga, Y., Hirata, T., Saito, A., Yoshino, O., Hirota, Y., Harada, M., Takemura, Y., Fujii, T., & Taketani, Y. (2011). High mobility group box 1 (HMGB1) levels in the placenta and in serum in preeclampsia [Journal Article; Research Support, Non-U.S. Gov't]. American Journal of Reproductive Immunology, 66(2), 143-148. http://doi.org/10.1111/j.1600-0897.2010.00975.x
[121] Li, W., Li, J., Ashok, M., Wu, R., Chen, D., Yang, L., Yang, H., Tracey, K. J., Wang, P., Sama, A. E., & Wang, H. (2007). A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1 [Journal Article; Research Support, N.I.H., Extramural]. Journal of Immunology, 178(6), 3856-3864. http://doi.org/10.4049/jimmunol.178.6.3856
[122] Nishiike, S., Hiramatsu, T., Shiraishi, M., Ueda, Y., & Tsuchida, H. (2013). Relationship between vascular reactivity and expression of HMGB1 in a rat model of septic aorta [Journal Article]. Journal of Anesthesia, 27(5), 684-692. http://doi.org/10.1007/s00540-013-1584-x
[123] Tang, D., Kang, R., Zeh, H. J., & Lotze, M. T. (2023). The multifunctional protein HMGB1: 50 years of discovery [Journal Article; Review]. Nature Reviews Immunology, 23(12), 824-841. http://doi.org/10.1038/s41577-023-00894-6
[124] Hou, W., Wei, X., Liang, J., Fang, P., Ma, C., Zhang, Q., & Gao, Y. (2021). HMGB1-Induced Hepatocyte Pyroptosis Expanding Inflammatory Responses Contributes to the Pathogenesis of Acute-on-Chronic Liver Failure (ACLF) [Journal Article]. Journal of Inflammation Research, 14, 7295-7313. http://doi.org/10.2147/JIR.S336626
[125] Jube, S., Rivera, Z. S., Bianchi, M. E., Powers, A., Wang, E., Pagano, I., Pass, H. I., Gaudino, G., Carbone, M., & Yang, H. (2012). Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cancer Research, 72(13), 3290-3301. http://doi.org/10.1158/0008-5472.CAN-11-3481
[126] Xue, J., Patergnani, S., Giorgi, C., Suarez, J., Goto, K., Bononi, A., Tanji, M., Novelli, F., Pastorino, S., Xu, R., Caroccia, N., Dogan, A. U., Pass, H. I., Tognon, M., Pinton, P., Gaudino, G., Mak, T. W., Carbone, M., & Yang, H. (2020). Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America, 117(41), 25543-25552. http://doi.org/10.1073/pnas.2007622117
[127] Mcdonald, K. A., Huang, H., Tohme, S., Loughran, P., Ferrero, K., Billiar, T., & Tsung, A. (2015). Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Molecular Medicine, 20(1), 639-648. http://doi.org/10.2119/molmed.2014.00076
[128] Chen, R., Huang, Y., Quan, J., Liu, J., Wang, H., Billiar, T. R., Lotze, M. T., Zeh, H. J., Kang, R., & Tang, D. (2020). HMGB1 as a potential biomarker and therapeutic target for severe COVID-19 [Journal Article]. Heliyon, 6(12), e05672. http://doi.org/10.1016/j.heliyon.2020.e05672
[129] Kumar S, N. R. M. V. (2020). Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronavirus Disease 2019 (COVID-19), 23-31.
[130] Li YG, W. X. L. Q. (2022). GLYCYRRHIZIN REGULATES HIPPOCAMPAL PATHOLOGICAL DAMAGE OF KAINIC ACID-INDUCED SEIZURES IN YOUNG RATS THROUGH HMGB1/TLR4/P-NF-KB PATHWAY. Acta Medica Mediterranea, 4(38), 2475-2479.
 Figures
Figures References
References Peer
Peer Information
InformationFigure 1. (A) Flow chart of HMGB1-related research screening process. (B) Description of HMGB1-related publications and citations. The blue bar represents the number and trend of articles, and the orange line represents the number and trend of citations.

Figure 2. (A) Countries/regions collaboration map. On the map of world administrative divisions, the lines between countries/regions represent collaborations, and more lines mean more frequent collaborations. (B) The chord diagram of collaboration between countries/regions. The arc is proportional to the total number of publications, the width of the arc is proportional to the total number of publications, and the width of the shading is proportional to collaborative publications between the two countries. (C) Analysis of the corresponding author's country. Blue represents the number of single country publications (SCP) and red represents the number of multiple collaborative publications (MCP). (Country analysis includes nations with a publication volume greater than 25.)

Figure 3. (A) Analysis of HMGB1-related collaborative network visualization of institutions in VOSviewer. Vosviewer automatically clusters all institutions into different colored clusters based on inter-institutional collaboration. Each dot represents an institution, the size of the dot reflects the number of publications, and the thickness of the line between the dots reflects the closeness of the collaboration between institutions. (B) Institutional analysis of published articles from 2012 to 2020. (Institutional analysis includes organizations with a publication volume greater than 15.)

Figure 4. (A) Analysis of HMGB1-related collaborative network visualization of authors in VOSviewer. VOSviewer divides authors into different colored clusters based on their collaboration status. Different colors represent closer collaboration relationships. Each dot in the graph represents an author, the size of the dot reflects the number of publications of the author, and the thickness of the line between the dots reflects the degree of cooperation. (B) Analysis of collaborative network visualization of co-cited authorship in VOSviewer. The analysis relates to one or more future works simultaneously citing two or more authors. Higher co-citation frequency denotes more concentrated academic attention and research. Vosviewer automatically clusters authors into different colored clusters based on their co-cited status, where each dot represents an author, the size of the dot reflects the co-cited strength, and the thickness of the line between the dots reflects the co-cited relationship of the two authors. (C) Analysis of authors that publish articles. The heat value for each keyword over the past five years is calculated by dividing the number of publications in the last five years by the total number of publications. (Author analysis includes authors with a publication volume greater than 10. Co-cited author analysis includes authors with citation counts greater than 175.)

Figure 5. (A) Analysis of HMGB1-related collaborative network visualization of journals in VOSviewer. The visualization in VOSviewer displays the journals and their relationship based on the proximity of the journals. Different dots represent different journals, the color reflects the corresponding clusters, and the connecting line indicates the closeness of the connection. (B) Analysis of collaborative network visualization of journal citations in VOSviewer. Different dots represent different journals, the colors reflect the corresponding clusters, and the lines indicate the closeness of the connections. (C) The dual-map overlay of journals. The distribution of themes, variations in citation trajectories, and changes in research centers are all displayed. The published journals are labeled on the left side of the double map, while the cited journals are labeled on the right. The context of the citation connection is indicated by the colored arrows pointing from the published journal to the referenced journal. (Journal analysis includes journals with a publication volume greater than 15. Co-cited journal analysis includes journals with citation counts greater than 500.)

Figure 6. (A) Analysis of HMGB1-related collaborative network visualization of keywords in VOSviewer. Clusters are formed based on keyword co-occurrence frequency, with colors distinguishing clusters. Node size indicates keyword frequency, and line thickness represents relationship strength. (Keyword analysis includes terms that appear more than 20.) (B) Thematic evaluation of the research field during 1990-2010. (C) Thematic evaluation of the research field during 2010-2015. (D) Thematic evaluation of the research field during 2015-2022. (E) Thematic evaluation of the research field during 2022-2024. (B-E) The thematic evaluation map visualizes four different periods of themes according to two dimensions (density and centrality). Motor themes are the keywords in the upper right corner of the first quadrant. Niche themes are located in the upper left (second quadrant), emerging or declining themes are in the lower left (third quadrant), and basic themes are in the lower right (fourth quadrant). The Motor Themes in the upper right quadrant represent directions that are relevant and already rapidly developing in the field of HMGB1, which is the focus of current research. The Basic Themes in the lower right quadrant represent topics that are relevant to the field of HMGB1 but are not yet well developed and may be the focus of future research in the field of HMGB1.

Figure 7. (A) Heatmap of keywords in temporal dimension. Each radial line corresponds to a specific keyword, while each concentric circle denotes a successive year. A color block with a more intense red indicates a higher frequency of the keyword's occurrence in that year's literature. (B) Heatmap of the top100 keywords. It depicts a keyword heatmap where each cell's color intensity correlates with the co-occurrence frequency of the two respective keywords it represents. A deeper red hue within a cell signifies a higher co-occurrence rate, indicating that extensive red areas are often indicative of a high degree of relevance between the associated keywords. (C) Annual heat map spanning from 1976 to 2024. The annual heat value for each keyword is calculated by dividing the citation count for that year by the total citations made in the same year. (D) Heat map illustrating keyword correlation. Keywords with high visibility are divided into different categories and distinguished by different colors.

Figure 8. (A) Association between the top highly cited references. Each node represents a highly cited paper, and arrows indicate citation relationships, pointing from the citing paper to the cited paper. The size of the nodes is proportional to the number of co-citations the paper has received. (B) Clustering of references based on the similarity between references, which shows the authors and the year of publications with an explosive increase in co-citations. The category with the most articles is 0, followed by 1, and so forth. (C) The top 25 references with the strongest citation bursts. The blue line indicates the timeline, and the red segments represent burst periods. A citation burst represent a sharp increase in the number of articles cited and some of the key issues in the field raised or addressed in the article.

[1] Goodwin, G. H., Sanders, C., & Johns, E. W. (1973). A new group of chromatin-associated proteins with a high content of acidic and basic amino acids [Journal Article]. Eur J Biochem, 38(1), 14-19. http://doi.org/10.1111/j.1432-1033.1973.tb03026.x
[2] Goodwin, G. H., & Johns, E. W. (1973). Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids [Journal Article]. Eur J Biochem, 40(1), 215-219. http://doi.org/10.1111/j.1432-1033.1973.tb03188.x
[3] Weir, H. M., Kraulis, P. J., Hill, C. S., Raine, A. R., Laue, E. D., & Thomas, J. O. (1993). Structure of the HMG box motif in the B-domain of HMG1 [Comparative Study; Journal Article; Research Support, Non-U.S. Gov't]. Embo Journal, 12(4), 1311-1319. http://doi.org/10.1002/j.1460-2075.1993.tb05776.x
[4] Balana, A. T., Mukherjee, A., Nagpal, H., Moon, S. P., Fierz, B., Vasquez, K. M., & Pratt, M. R. (2021). O-GlcNAcylation of High Mobility Group Box 1 (HMGB1) Alters Its DNA Binding and DNA Damage Processing Activities [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of the American Chemical Society, 143(39), 16030-16040. http://doi.org/10.1021/jacs.1c06192
[5] Polyanichko A, W. H. (2010). Structural organization of DNA-protein complexes of chromatin studied by vibrational and electronic circular dichroism. Spectroscopy-an International Journal, 3-4(24), 239-244.
[6] Song, M. J., Hwang, S., Wong, W., Round, J., Martinez-Guzman, D., Turpaz, Y., Liang, J., Wong, B., Johnson, R. C., Carey, M., & Sun, R. (2004). The DNA architectural protein HMGB1 facilitates RTA-mediated viral gene expression in gamma-2 herpesviruses [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. Journal of Virology, 78(23), 12940-12950. http://doi.org/10.1128/JVI.78.23.12940-12950.2004
[7] Ueda, T., Chou, H., Kawase, T., Shirakawa, H., & Yoshida, M. (2004). Acidic C-tail of HMGB1 is required for its target binding to nucleosome linker DNA and transcription stimulation [Journal Article; Research Support, Non-U.S. Gov't]. Biochemistry, 43(30), 9901-9908. http://doi.org/10.1021/bi035975l
[8] Wu, X. J., Chen, Y. Y., Guo, W. W., Li, T., Dong, H. B., Wang, W., Xie, M., Ma, G. L., & Pei, D. S. (2020). HMGB1 regulates SNAI1 during NSCLC metastasis, both directly, through transcriptional activation, and indirectly, in a RSF1-IT2-dependent manner [Journal Article; Research Support, Non-U.S. Gov't]. Molecular Oncology, 14(6), 1348-1364. http://doi.org/10.1002/1878-0261.12691
[9] Zhan, Z., Li, Q., Wu, P., Ye, Y., Tseng, H. Y., Zhang, L., & Zhang, X. D. (2012). Autophagy-mediated HMGB1 release antagonizes apoptosis of gastric cancer cells induced by vincristine via transcriptional regulation of Mcl-1 [Journal Article; Research Support, Non-U.S. Gov't]. Autophagy, 8(1), 109-121. http://doi.org/10.4161/auto.8.1.18319
[10] Chen, R., Kang, R., & Tang, D. (2022). The mechanism of HMGB1 secretion and release [Journal Article; Research Support, Non-U.S. Gov't; Review]. Experimental and Molecular Medicine, 54(2), 91-102. http://doi.org/10.1038/s12276-022-00736-w
[11] Kim, Y. H., Kwak, M. S., Park, J. B., Lee, S. A., Choi, J. E., Cho, H. S., & Shin, J. S. (2016). N-linked glycosylation plays a crucial role in the secretion of HMGB1 [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cell Science, 129(1), 29-38. http://doi.org/10.1242/jcs.176412
[12] Lu, B., Wang, H., Andersson, U., & Tracey, K. J. (2013). Regulation of HMGB1 release by inflammasomes [Journal Article; Review]. Protein & Cell, 4(3), 163-167. http://doi.org/10.1007/s13238-012-2118-2
[13] Andersson, U., Tracey, K. J., & Yang, H. (2021). Post-Translational Modification of HMGB1 Disulfide Bonds in Stimulating and Inhibiting Inflammation [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cells, 10(12) http://doi.org/10.3390/cells10123323
[14] Watanabe, H., & Son, M. (2021). The Immune Tolerance Role of the HMGB1-RAGE Axis [Journal Article; Research Support, N.I.H., Extramural; Review]. Cells, 10(3) http://doi.org/10.3390/cells10030564
[15] Liu, X., Lu, B., Fu, J., Zhu, X., Song, E., & Song, Y. (2021). Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Hazardous Materials, 404(Pt B), 124050. http://doi.org/10.1016/j.jhazmat.2020.124050
[16] Mohamed, M. E., Kandeel, M., Abd, E. H., El-Beltagi, H. S., & Younis, N. S. (2022). The Protective Effect of Anethole against Renal Ischemia/Reperfusion: The Role of the TLR2,4/MYD88/NFkappaB Pathway [Journal Article]. Antioxidants, 11(3) http://doi.org/10.3390/antiox11030535
[17] Denning, N. L., Aziz, M., Gurien, S. D., & Wang, P. (2019). DAMPs and NETs in Sepsis [Journal Article; Research Support, N.I.H., Extramural; Review]. Frontiers in Immunology, 10, 2536. http://doi.org/10.3389/fimmu.2019.02536
[18] Rani, M., Nicholson, S. E., Zhang, Q., & Schwacha, M. G. (2017). Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation [Journal Article]. Burns, 43(2), 297-303. http://doi.org/10.1016/j.burns.2016.10.001
[19] Lee, S. A., Kwak, M. S., Kim, S., & Shin, J. S. (2014). The role of high mobility group box 1 in innate immunity [Journal Article; Research Support, Non-U.S. Gov't; Review]. Yonsei Medical Journal, 55(5), 1165-1176. http://doi.org/10.3349/ymj.2014.55.5.1165
[20] Xu, K., Ren, X., Ju, B., Aihaiti, Y., Cai, Y., Zhang, Y., He, L., & Wang, J. (2020). Clinical markers combined with HMGB1 polymorphisms to predict efficacy of conventional DMARDs in rheumatoid arthritis patients [Journal Article; Research Support, Non-U.S. Gov't]. Clinical Immunology, 221, 108592. http://doi.org/10.1016/j.clim.2020.108592
[21] Wu, R., Wang, N., Comish, P. B., Tang, D., & Kang, R. (2021). Inflammasome-Dependent Coagulation Activation in Sepsis [Journal Article; Review]. Frontiers in Immunology, 12, 641750. http://doi.org/10.3389/fimmu.2021.641750
[22] Foglio, E., Pellegrini, L., Russo, M. A., & Limana, F. (2022). HMGB1-Mediated Activation of the Inflammatory-Reparative Response Following Myocardial Infarction [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cells, 11(2) http://doi.org/10.3390/cells11020216
[23] Meng, X., Su, W., Tao, X., Sun, M., Ying, R., Wei, W., & Wang, B. (2018). Oxidation Prevents HMGB1 Inhibition on PDGF-Induced Differentiation of Multipotent Vascular Stem Cells to Smooth Muscle Cells: A Possible Mechanism Linking Oxidative Stress to Atherosclerosis [Journal Article]. Biomed Research International, 2018, 4019814. http://doi.org/10.1155/2018/4019814
[24] Hemmer, S., Senger, S., Griessenauer, C. J., Simgen, A., Oertel, J., Geisel, J., & Hendrix, P. (2022). Admission serum high mobility group box 1 (HMGB1) protein predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage [Journal Article; Observational Study]. Neurosurgical Review, 45(1), 807-817. http://doi.org/10.1007/s10143-021-01607-0
[25] Yang, L., Zhou, L., Wang, X., Wang, W., & Wang, J. (2020). Inhibition of HMGB1 involved in the protective of salidroside on liver injury in diabetes mice [Journal Article]. International Immunopharmacology, 89(Pt A), 106987. http://doi.org/10.1016/j.intimp.2020.106987
[26] Tanaka, A., Ito, T., Kibata, K., Inagaki-Katashiba, N., Amuro, H., Nishizawa, T., Son, Y., Ozaki, Y., & Nomura, S. (2019). Serum high-mobility group box 1 is correlated with interferon-alpha and may predict disease activity in patients with systemic lupus erythematosus [Journal Article]. Lupus, 28(9), 1120-1127. http://doi.org/10.1177/0961203319862865
[27] Ho, W. I., Hu, Y., Cheng, C. W., Wei, R., Yang, J., Li, N., Au, K. W., Tse, Y. L., Wang, Q., Ng, K. M., Esteban, M. A., & Tse, H. F. (2022). Liposome-encapsulated curcumin attenuates HMGB1-mediated hepatic inflammation and fibrosis in a murine model of Wilson's disease [Journal Article]. Biomedicine & Pharmacotherapy, 152, 113197. http://doi.org/10.1016/j.biopha.2022.113197
[28] Sui, H., Luo, M., Miao, Y., Cheng, W., Wen, S., Zhao, B., Li, Y., Qiao, Z., Liu, Y., & Xu, C. (2020). Cystic fibrosis transmembrane conductance regulator ameliorates lipopolysaccharide-induced acute lung injury by inhibiting autophagy through PI3K/AKT/mTOR pathway in mice [Journal Article; Research Support, Non-U.S. Gov't]. Respiratory Physiology & Neurobiology, 273, 103338. http://doi.org/10.1016/j.resp.2019.103338
[29] Jankauskaite, L., Malinauskas, M., & Mickeviciute, G. C. (2022). HMGB1: A Potential Target of Nervus Vagus Stimulation in Pediatric SARS-CoV-2-Induced ALI/ARDS [Journal Article]. Frontiers in Pediatrics, 10, 884539. http://doi.org/10.3389/fped.2022.884539
[30] Teo, H. S. A., Schlichtner, S., Yasinska, I. M., Sakhnevych, S. S., Fiedler, W., Wellbrock, J., Berger, S. M., Klenova, E., Gibbs, B. F., Fasler-Kan, E., & Sumbayev, V. V. (2021). High Mobility Group Box 1 (HMGB1) Induces Toll-Like Receptor 4-Mediated Production of the Immunosuppressive Protein Galectin-9 in Human Cancer Cells [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 12, 675731. http://doi.org/10.3389/fimmu.2021.675731
[31] Simon, D. W., Aneja, R. K., Alexander, H., Bell, M. J., Bayir, H., Kochanek, P. M., & Clark, R. (2018). Minocycline Attenuates High Mobility Group Box 1 Translocation, Microglial Activation, and Thalamic Neurodegeneration after Traumatic Brain Injury in Post-Natal Day 17 Rats [Journal Article; Research Support, N.I.H., Extramural]. Journal of Neurotrauma, 35(1), 130-138. http://doi.org/10.1089/neu.2017.5093
[32] Hicks, D., Wouters, P., Waltman, L., de Rijcke, S., & Rafols, I. (2015). Bibliometrics: The Leiden Manifesto for research metrics [Historical Article; Journal Article]. Nature, 520(7548), 429-431. http://doi.org/10.1038/520429a
[33] Deng, P., Shi, H., Pan, X., Liang, H., Wang, S., Wu, J., Zhang, W., Huang, F., Sun, X., Zhu, H., & Chen, Z. (2022). Worldwide Research Trends on Diabetic Foot Ulcers (2004-2020): Suggestions for Researchers [Journal Article]. Journal of Diabetes Research, 2022, 7991031. http://doi.org/10.1155/2022/7991031
[34] Ninkov, A., Frank, J. R., & Maggio, L. A. (2022). Bibliometrics: Methods for studying academic publishing [Journal Article]. Perspectives On Medical Education, 11(3), 173-176. http://doi.org/10.1007/s40037-021-00695-4
[35] Shi, X., Wang, S., Wu, Y., Li, Q., Zhang, T., Min, K., Feng, D., Liu, M., Wei, J., Zhu, L., Mo, W., Xiao, Z., Yang, H., Chen, Y., & Lv, X. (2022). A Bibliometric Analysis of the Innate Immune DNA Sensing cGAS-STING Pathway from 2013 to 2021 [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 13, 916383. http://doi.org/10.3389/fimmu.2022.916383
[36] Ma, D., Yang, B., Guan, B., Song, L., Liu, Q., Fan, Y., Zhao, L., Wang, T., Zhang, Z., Gao, Z., Li, S., & Xu, H. (2021). A Bibliometric Analysis of Pyroptosis From 2001 to 2021 [Journal Article; Research Support, Non-U.S. Gov't]. Frontiers in Immunology, 12, 731933. http://doi.org/10.3389/fimmu.2021.731933
[37] Dong, X., Tan, Y., Zhuang, D., Hu, T., & Zhao, M. (2022). Global Characteristics and Trends in Research on Ferroptosis: A Data-Driven Bibliometric Study [Journal Article; Review]. Oxidative Medicine and Cellular Longevity, 2022, 8661864. http://doi.org/10.1155/2022/8661864
[38] Chen, C. (2006). CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. Journal of the American Society for Information Science and Technology, 57(3), 359-377. http://doi.org/10.1002/asi.20317
[39] van Eck, N. J., & Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping [Journal Article]. Scientometrics, 84(2), 523-538. http://doi.org/10.1007/s11192-009-0146-3
[40] Aria, M., & Cuccurullo, C. (2018). bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. Journal of Informetrics, 11(4), 959-975.
[41] Scaffidi, P., Misteli, T., & Bianchi, M. E. (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation [Journal Article; Research Support, Non-U.S. Gov't]. Nature, 418(6894), 191-195. http://doi.org/10.1038/nature00858
[42] Park, J. S., Svetkauskaite, D., He, Q., Kim, J. Y., Strassheim, D., Ishizaka, A., & Abraham, E. (2004). Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein [Journal Article; Research Support, U.S. Gov't, P.H.S.]. Journal of Biological Chemistry, 279(9), 7370-7377. http://doi.org/10.1074/jbc.M306793200
[43] Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., Huang, J., Yu, Y., Fan, X. G., Yan, Z., Sun, X., Wang, H., Wang, Q., Tsung, A., Billiar, T. R., Zeh, H. R., Lotze, M. T., & Tang, D. (2014). HMGB1 in health and disease [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Molecular Aspects of Medicine, 40, 1-116. http://doi.org/10.1016/j.mam.2014.05.001
[44] Chen, R., Kang, R., & Tang, D. (2022). The mechanism of HMGB1 secretion and release [Journal Article; Research Support, Non-U.S. Gov't; Review]. Experimental and Molecular Medicine, 54(2), 91-102. http://doi.org/10.1038/s12276-022-00736-w
[45] Lee, S. Y., Ju, M. K., Jeon, H. M., Jeong, E. K., Lee, Y. J., Kim, C. H., Park, H. G., Han, S. I., & Kang, H. S. (2018). Regulation of Tumor Progression by Programmed Necrosis [Journal Article; Review]. Oxidative Medicine and Cellular Longevity, 2018, 3537471. http://doi.org/10.1155/2018/3537471
[46] Stelmasiak, M., Mikaszewska-Sokolewicz, M., NiewiNski, G., BaLan, B. J., & SLotwiNski, R. (2020). The soluble tumor necrosis factor receptor 1 as a potential early diagnostic and prognostic markers in intensive care unit patients with severe infections [Journal Article]. Central European Journal of Immunology, 45(2), 160-169. http://doi.org/10.5114/ceji.2020.97903
[47] Tang, D., Kang, R., Livesey, K. M., Cheh, C. W., Farkas, A., Loughran, P., Hoppe, G., Bianchi, M. E., Tracey, K. J., Zeh, H. R., & Lotze, M. T. (2010). Endogenous HMGB1 regulates autophagy [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of Cell Biology, 190(5), 881-892. http://doi.org/10.1083/jcb.200911078
[48] Tang, D., Kang, R., Livesey, K. M., Kroemer, G., Billiar, T. R., Van Houten, B., Zeh, H. R., & Lotze, M. T. (2011). High-mobility group box 1 is essential for mitochondrial quality control [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cell Metabolism, 13(6), 701-711. http://doi.org/10.1016/j.cmet.2011.04.008
[49] Kang, R., Zhang, Q., Zeh, H. R., Lotze, M. T., & Tang, D. (2013). HMGB1 in cancer: good, bad, or both? [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Clinical Cancer Research, 19(15), 4046-4057. http://doi.org/10.1158/1078-0432.CCR-13-0495
[50] Livesey, K. M., Kang, R., Vernon, P., Buchser, W., Loughran, P., Watkins, S. C., Zhang, L., Manfredi, J. J., Zeh, H. R., Li, L., Lotze, M. T., & Tang, D. (2012). p53/HMGB1 complexes regulate autophagy and apoptosis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cancer Research, 72(8), 1996-2005. http://doi.org/10.1158/0008-5472.CAN-11-2291
[51] Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., Huang, J., Yu, Y., Fan, X. G., Yan, Z., Sun, X., Wang, H., Wang, Q., Tsung, A., Billiar, T. R., Zeh, H. R., Lotze, M. T., & Tang, D. (2014). HMGB1 in health and disease [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Molecular Aspects of Medicine, 40, 1-116. http://doi.org/10.1016/j.mam.2014.05.001
[52] Huang, J., Ni, J., Liu, K., Yu, Y., Xie, M., Kang, R., Vernon, P., Cao, L., & Tang, D. (2012). HMGB1 promotes drug resistance in osteosarcoma [Journal Article; Research Support, Non-U.S. Gov't]. Cancer Research, 72(1), 230-238. http://doi.org/10.1158/0008-5472.CAN-11-2001
[53] Liu, L., Yang, M., Kang, R., Wang, Z., Zhao, Y., Yu, Y., Xie, M., Yin, X., Livesey, K. M., Lotze, M. T., Tang, D., & Cao, L. (2011). HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells [Journal Article; Research Support, Non-U.S. Gov't]. Leukemia, 25(1), 23-31. http://doi.org/10.1038/leu.2010.225
[54] Luo, Y., Yoneda, J., Ohmori, H., Sasaki, T., Shimbo, K., Eto, S., Kato, Y., Miyano, H., Kobayashi, T., Sasahira, T., Chihara, Y., & Kuniyasu, H. (2014). Cancer usurps skeletal muscle as an energy repository [Journal Article; Research Support, Non-U.S. Gov't]. Cancer Research, 74(1), 330-340. http://doi.org/10.1158/0008-5472.CAN-13-1052
[55] Kang, R., Zeh, H. J., Lotze, M. T., & Tang, D. (2011). The Beclin 1 network regulates autophagy and apoptosis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Cell Death and Differentiation, 18(4), 571-580. http://doi.org/10.1038/cdd.2010.191
[56] Liu, B., Gan, X., Zhao, Y., Gao, J., & Yu, H. (2021). Inhibition of HMGB1 reduced high glucose-induced BMSCs apoptosis via activation of AMPK and regulation of mitochondrial functions [Journal Article]. Journal of Physiology and Biochemistry, 77(2), 227-235. http://doi.org/10.1007/s13105-021-00784-2
[57] Brezniceanu, M. L., Volp, K., Bosser, S., Solbach, C., Lichter, P., Joos, S., & Zornig, M. (2003). HMGB1 inhibits cell death in yeast and mammalian cells and is abundantly expressed in human breast carcinoma [Journal Article]. Faseb Journal, 17(10), 1295-1297. http://doi.org/10.1096/fj.02-0621fje
[58] Jiang, H., Hu, X., Zhang, H., & Li, W. (2017). Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression [Journal Article]. Radiation Oncology, 12(1), 65. http://doi.org/10.1186/s13014-017-0802-3
[59] Zhi, S. M., Fang, G. X., Xie, X. M., Liu, L. H., Yan, J., Liu, D. B., & Yu, H. Y. (2020). Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling [Journal Article; Research Support, Non-U.S. Gov't]. European Review for Medical and Pharmacological Sciences, 24(3), 1524-1536. http://doi.org/10.26355/eurrev_202002_20211
[60] Huo, J., Shen, Y., Zhang, Y., & Shen, L. (2022). BI 2536 induces gasdermin E-dependent pyroptosis in ovarian cancer [Journal Article]. Frontiers in Oncology, 12, 963928. http://doi.org/10.3389/fonc.2022.963928
[61] Zeng, C. Y., Li, C. G., Shu, J. X., Xu, L. H., Ouyang, D. Y., Mai, F. Y., Zeng, Q. Z., Zhang, C. C., Li, R. M., & He, X. H. (2019). ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages [Journal Article; Research Support, Non-U.S. Gov't]. Apoptosis, 24(9-10), 703-717. http://doi.org/10.1007/s10495-019-01551-x
[62] Kamo N, K. B. G. A. (2013). ASC/Caspase-1/IL-1 beta Signaling Triggers Inflammatory Responses by Promoting HMGB1 Induction in Liver Ischemia/Reperfusion Injury. Hepatology, 1(58), 351-362.
[63] Li, Y., Yuan, Y., Huang, Z. X., Chen, H., Lan, R., Wang, Z., Lai, K., Chen, H., Chen, Z., Zou, Z., Ma, H. B., Lan, H. Y., Mak, T. W., & Xu, Y. (2021). GSDME-mediated pyroptosis promotes inflammation and fibrosis in obstructive nephropathy [Journal Article; Research Support, Non-U.S. Gov't]. Cell Death and Differentiation, 28(8), 2333-2350. http://doi.org/10.1038/s41418-021-00755-6
[64] Chen, X., Yu, C., Kang, R., Kroemer, G., & Tang, D. (2021). Cellular degradation systems in ferroptosis [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cell Death and Differentiation, 28(4), 1135-1148. http://doi.org/10.1038/s41418-020-00728-1
[65] Lin, C., Hu, R., Sun, F., & Liang, W. (2022). Ferroptosis-based molecular prognostic model for adrenocortical carcinoma based on least absolute shrinkage and selection operator regression [Journal Article]. Journal of Clinical Laboratory Analysis, 36(6), e24465. http://doi.org/10.1002/jcla.24465
[66] Hu, N., Bai, L., Dai, E., Han, L., Kang, R., Li, H., & Tang, D. (2021). Pirin is a nuclear redox-sensitive modulator of autophagy-dependent ferroptosis [Journal Article]. Biochemical and Biophysical Research Communications, 536, 100-106. http://doi.org/10.1016/j.bbrc.2020.12.066
[67] Wen, Q., Liu, J., Kang, R., Zhou, B., & Tang, D. (2019). The release and activity of HMGB1 in ferroptosis [Journal Article; Research Support, Non-U.S. Gov't]. Biochemical and Biophysical Research Communications, 510(2), 278-283. http://doi.org/10.1016/j.bbrc.2019.01.090
[68] He, C., Sun, S., Zhang, Y., Xie, F., & Li, S. (2021). The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer [Journal Article; Research Support, Non-U.S. Gov't]. OncoImmunology, 10(1), 1897295. http://doi.org/10.1080/2162402X.2021.1897295
[69] Cebrian, M. J., Bauden, M., Andersson, R., Holdenrieder, S., & Ansari, D. (2016). Paradoxical Role of HMGB1 in Pancreatic Cancer: Tumor Suppressor or Tumor Promoter? [Journal Article; Review]. Anticancer Research, 36(9), 4381-4389. http://doi.org/10.21873/anticanres.10981
[70] Dong, H., Zhang, L., & Liu, S. (2022). Targeting HMGB1: An available Therapeutic Strategy for Breast Cancer Therapy [Journal Article; Research Support, Non-U.S. Gov't; Review]. International Journal of Biological Sciences, 18(8), 3421-3434. http://doi.org/10.7150/ijbs.73504
[71] Wang, H., Zhang, S., Zhao, H., Qin, H., Zhang, J., Dong, J., Zhang, H., Liu, X., Zhao, Z., Zhao, Y., Shao, M., Wu, F., & Zhang, W. (2020). Carbon Monoxide Inhibits the Expression of Proteins Associated with Intestinal Mucosal Pyroptosis in a Rat Model of Sepsis Induced by Cecal Ligation and Puncture [Journal Article]. Medical Science Monitor, 26, e920668. http://doi.org/10.12659/MSM.920668
[72] Chen, G., Hou, Y., Li, X., Pan, R., & Zhao, D. (2021). Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-kappaB pathway and NLRP3 inflammasome [Journal Article]. International Immunopharmacology, 96, 107623. http://doi.org/10.1016/j.intimp.2021.107623
[73] Li, Y., Zhu, H., Pan, L., Zhang, B., & Che, H. (2021). microRNA-103a-3p confers protection against lipopolysaccharide-induced sepsis and consequent multiple organ dysfunction syndrome by targeting HMGB1 [Journal Article]. Infection Genetics and Evolution, 89, 104681. http://doi.org/10.1016/j.meegid.2020.104681
[74] Kruger, B., Krick, S., Dhillon, N., Lerner, S. M., Ames, S., Bromberg, J. S., Lin, M., Walsh, L., Vella, J., Fischereder, M., Kramer, B. K., Colvin, R. B., Heeger, P. S., Murphy, B. T., & Schroppel, B. (2009). Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Proceedings of the National Academy of Sciences of the United States of America, 106(9), 3390-3395. http://doi.org/10.1073/pnas.0810169106
[75] Panisello-Rosello, A., Verde, E., Lopez, A., Flores, M., Folch-Puy, E., Rolo, A., Palmeira, C., Hotter, G., Carbonell, T., Adam, R., & Rosello-Catafau, J. (2018). Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK [Journal Article]. International Journal of Molecular Sciences, 19(2) http://doi.org/10.3390/ijms19020348
[76] Manti, S., Harford, T. J., Salpietro, C., Rezaee, F., & Piedimonte, G. (2018). Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus [Journal Article; Research Support, N.I.H., Extramural]. Pediatric Research, 83(5), 1049-1056. http://doi.org/10.1038/pr.2018.6
[77] Wyganowska-Swiatkowska, M., Nohawica, M., Grocholewicz, K., & Nowak, G. (2020). Influence of Herbal Medicines on HMGB1 Release, SARS-CoV-2 Viral Attachment, Acute Respiratory Failure, and Sepsis. A Literature Review [Journal Article; Review]. International Journal of Molecular Sciences, 21(13) http://doi.org/10.3390/ijms21134639
[78] Ogura, Y., Fukuchi, K., Morimoto, H., Yuki, T., Otsuka, M., Shimauchi, T., Honda, T., & Tokura, Y. (2022). Elevation of circulating neutrophil extracellular traps, interleukin (IL)-8, IL-22, and vascular endothelial growth factor in patients with venomous snake mamushi (Gloydius blomhoffii) bites [Journal Article]. Journal of Dermatology, 49(1), 124-132. http://doi.org/10.1111/1346-8138.16181
[79] Wahid, A., Chen, W., Wang, X., & Tang, X. (2021). High-mobility group box 1 serves as an inflammation driver of cardiovascular disease [Journal Article; Review]. Biomedicine & Pharmacotherapy, 139, 111555. http://doi.org/10.1016/j.biopha.2021.111555
[80] Kalinina, N., Agrotis, A., Antropova, Y., DiVitto, G., Kanellakis, P., Kostolias, G., Ilyinskaya, O., Tararak, E., & Bobik, A. (2004). Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines [Journal Article]. Arteriosclerosis Thrombosis and Vascular Biology, 24(12), 2320-2325. http://doi.org/10.1161/01.ATV.0000145573.36113.8a
[81] Liu, M., Yu, Y., Jiang, H., Zhang, L., Zhang, P. P., Yu, P., Jia, J. G., Chen, R. Z., Zou, Y. Z., & Ge, J. B. (2013). Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis [Journal Article; Research Support, Non-U.S. Gov't]. Acta Pharmacologica Sinica, 34(6), 830-836. http://doi.org/10.1038/aps.2013.8
[82] Feng, Y., Ke, J., Cao, P., Deng, M., Li, J., Cai, H., Meng, Q., Li, Y., & Long, X. (2018). HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cellular and Molecular Medicine, 22(2), 1283-1291. http://doi.org/10.1111/jcmm.13410
[83] van Beijnum, J. R., Nowak-Sliwinska, P., van den Boezem, E., Hautvast, P., Buurman, W. A., & Griffioen, A. W. (2013). Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. Oncogene, 32(3), 363-374. http://doi.org/10.1038/onc.2012.49
[84] Rapoport, B. L., Steel, H. C., Theron, A. J., Heyman, L., Smit, T., Ramdas, Y., & Anderson, R. (2020). High Mobility Group Box 1 in Human Cancer [Journal Article; Review]. Cells, 9(7) http://doi.org/10.3390/cells9071664
[85] Wang, J. D., Wang, Y. Y., Lin, S. Y., Chang, C. Y., Li, J. R., Huang, S. W., Chen, W. Y., Liao, S. L., & Chen, C. J. (2021). Exosomal HMGB1 Promoted Cancer Malignancy [Journal Article]. Cancers, 13(4) http://doi.org/10.3390/cancers13040877
[86] He, S. J., Cheng, J., Feng, X., Yu, Y., Tian, L., & Huang, Q. (2017). The dual role and therapeutic potential of high-mobility group box 1 in cancer [Journal Article; Review]. Oncotarget, 8(38), 64534-64550. http://doi.org/10.18632/oncotarget.17885
[87] Xu, T., Jiang, L., & Wang, Z. (2019). The progression of HMGB1-induced autophagy in cancer biology [Journal Article; Review]. OncoTargets and Therapy, 12, 365-377. http://doi.org/10.2147/OTT.S185876
[88] Mandke, P., & Vasquez, K. M. (2019). Interactions of high mobility group box protein 1 (HMGB1) with nucleic acids: Implications in DNA repair and immune responses [Journal Article; Research Support, N.I.H., Extramural; Review]. Dna Repair, 83, 102701. http://doi.org/10.1016/j.dnarep.2019.102701
[89] Goldstein, R. S., Bruchfeld, A., Yang, L., Qureshi, A. R., Gallowitsch-Puerta, M., Patel, N. B., Huston, B. J., Chavan, S., Rosas-Ballina, M., Gregersen, P. K., Czura, C. J., Sloan, R. P., Sama, A. E., & Tracey, K. J. (2007). Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis [Comparative Study; Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Molecular Medicine, 13(3-4), 210-215. http://doi.org/10.2119/2006-00108.Goldstein
[90] Kaur, I., Behl, T., Bungau, S., Kumar, A., Mehta, V., Setia, D., Uddin, M. S., Zengin, G., Aleya, L., & Arora, S. (2020). Exploring the therapeutic promise of targeting HMGB1 in rheumatoid arthritis [Journal Article; Review]. Life Sciences, 258, 118164. http://doi.org/10.1016/j.lfs.2020.118164
[91] Garcia-Arnandis, I., Guillen, M. I., Gomar, F., Pelletier, J. P., Martel-Pelletier, J., & Alcaraz, M. J. (2010). High mobility group box 1 potentiates the pro-inflammatory effects of interleukin-1beta in osteoarthritic synoviocytes [Journal Article; Research Support, Non-U.S. Gov't]. Arthritis Research & Therapy, 12(4), R165. http://doi.org/10.1186/ar3124
[92] Qin, Y., Chen, Y., Wang, W., Wang, Z., Tang, G., Zhang, P., He, Z., Liu, Y., Dai, S. M., & Shen, Q. (2014). HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype [Journal Article; Research Support, Non-U.S. Gov't]. Cell Death & Disease, 5(2), e1077. http://doi.org/10.1038/cddis.2014.48
[93] Li, R., Jia, F., Ren, K., Luo, M., Min, X., Xiao, S., & Xia, Y. (2021). Fibroblast growth factor inducible 14 signaling facilitates anti-dsDNA IgG penetration into mesangial cells [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Cellular Physiology, 236(1), 249-259. http://doi.org/10.1002/jcp.29838
[94] Kuwano, A., Kohjima, M., Suzuki, H., Yamasaki, A., Ohashi, T., Imoto, K., Kurokawa, M., Morita, Y., Kato, M., & Ogawa, Y. (2019). Recombinant human soluble thrombomodulin ameliorates acetaminophen-induced liver toxicity in mice [Journal Article]. Experimental and Therapeutic Medicine, 18(2), 1323-1330. http://doi.org/10.3892/etm.2019.7665
[95] Regan, J. K., Kannan, P. S., Kemp, M. W., Kramer, B. W., Newnham, J. P., Jobe, A. H., & Kallapur, S. G. (2016). Damage-Associated Molecular Pattern and Fetal Membrane Vascular Injury and Collagen Disorganization in Lipopolysaccharide-Induced Intra-amniotic Inflammation in Fetal Sheep [Journal Article; Research Support, N.I.H., Extramural]. Reproductive Sciences, 23(1), 69-80. http://doi.org/10.1177/1933719115594014
[96] Chen, Y., Qiao, F., Zhao, Y., Wang, Y., & Liu, G. (2015). HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose [Journal Article; Research Support, Non-U.S. Gov't]. International Journal of Clinical and Experimental Pathology, 8(6), 6683-6691.
[97] Wang, X., Feng, C., Qiao, Y., & Zhao, X. (2018). Sigma 1 receptor mediated HMGB1 expression in spinal cord is involved in the development of diabetic neuropathic pain [Journal Article; Research Support, Non-U.S. Gov't]. Neuroscience Letters, 668, 164-168. http://doi.org/10.1016/j.neulet.2018.02.002
[98] Gao, T. L., Yuan, X. T., Yang, D., Dai, H. L., Wang, W. J., Peng, X., Shao, H. J., Jin, Z. F., & Fu, Z. J. (2012). Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury [Comparative Study; Journal Article; Research Support, Non-U.S. Gov't]. Journal of Trauma and Acute Care Surgery, 72(3), 643-649. http://doi.org/10.1097/TA.0b013e31823c54a6
[99] Okuma, Y., Liu, K., Wake, H., Zhang, J., Maruo, T., Date, I., Yoshino, T., Ohtsuka, A., Otani, N., Tomura, S., Shima, K., Yamamoto, Y., Yamamoto, H., Takahashi, H. K., Mori, S., & Nishibori, M. (2012). Anti-high mobility group box-1 antibody therapy for traumatic brain injury [Journal Article; Research Support, Non-U.S. Gov't]. Annals of Neurology, 72(3), 373-384. http://doi.org/10.1002/ana.23602
[100] Haruma, J., Teshigawara, K., Hishikawa, T., Wang, D., Liu, K., Wake, H., Mori, S., Takahashi, H. K., Sugiu, K., Date, I., & Nishibori, M. (2016). Anti-high mobility group box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats [Journal Article; Research Support, Non-U.S. Gov't]. Scientific Reports, 6, 37755. http://doi.org/10.1038/srep37755
[101] Fujita, K., Motoki, K., Tagawa, K., Chen, X., Hama, H., Nakajima, K., Homma, H., Tamura, T., Watanabe, H., Katsuno, M., Matsumi, C., Kajikawa, M., Saito, T., Saido, T., Sobue, G., Miyawaki, A., & Okazawa, H. (2016). HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer's disease [Journal Article; Research Support, Non-U.S. Gov't]. Scientific Reports, 6, 31895. http://doi.org/10.1038/srep31895
[102] Angelopoulou, E., Paudel, Y. N., & Piperi, C. (2020). Exploring the role of high-mobility group box 1 (HMGB1) protein in the pathogenesis of Huntington's disease [Journal Article; Research Support, Non-U.S. Gov't; Review]. Journal of Molecular Medicine-Jmm, 98(3), 325-334. http://doi.org/10.1007/s00109-020-01885-z
[103] Ren, Q., Jiang, X., Paudel, Y. N., Gao, X., Gao, D., Zhang, P., Sheng, W., Shang, X., Liu, K., Zhang, X., & Jin, M. (2022). Co-treatment with natural HMGB1 inhibitor Glycyrrhizin exerts neuroprotection and reverses Parkinson's disease like pathology in Zebrafish [Journal Article]. Journal of Ethnopharmacology, 292, 115234. http://doi.org/10.1016/j.jep.2022.115234
[104] Paudel, Y. N., Angelopoulou, E., Piperi, C., Othman, I., & Shaikh, M. F. (2020). Implication of HMGB1 signaling pathways in Amyotrophic lateral sclerosis (ALS): From molecular mechanisms to pre-clinical results [Journal Article; Research Support, Non-U.S. Gov't; Review]. Pharmacological Research, 156, 104792. http://doi.org/10.1016/j.phrs.2020.104792
[105] Kleen, J. K., & Holmes, G. L. (2010). Taming TLR4 may ease seizures [Comment; News]. Nature Medicine, 16(4), 369-370. http://doi.org/10.1038/nm0410-369
[106] Maroso, M., Balosso, S., Ravizza, T., Liu, J., Bianchi, M. E., & Vezzani, A. (2011). Interleukin-1 type 1 receptor/Toll-like receptor signalling in epilepsy: the importance of IL-1beta and high-mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. Journal of Internal Medicine, 270(4), 319-326. http://doi.org/10.1111/j.1365-2796.2011.02431.x
[107] Vezzani, A., Maroso, M., Balosso, S., Sanchez, M. A., & Bartfai, T. (2011). IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures [Journal Article; Review]. Brain Behavior and Immunity, 25(7), 1281-1289. http://doi.org/10.1016/j.bbi.2011.03.018
[108] Zurolo, E., Iyer, A., Maroso, M., Carbonell, C., Anink, J. J., Ravizza, T., Fluiter, K., Spliet, W. G., van Rijen, P. C., Vezzani, A., & Aronica, E. (2011). Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development [Journal Article; Research Support, Non-U.S. Gov't]. Brain, 134(Pt 4), 1015-1032. http://doi.org/10.1093/brain/awr032
[109] Balosso, S., Liu, J., Bianchi, M. E., & Vezzani, A. (2014). Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons [Journal Article; Research Support, Non-U.S. Gov't]. Antioxidants & Redox Signaling, 21(12), 1726-1740. http://doi.org/10.1089/ars.2013.5349
[110] Feldman, P., Due, M. R., Ripsch, M. S., Khanna, R., & White, F. A. (2012). The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. Journal of Neuroinflammation, 9, 180. http://doi.org/10.1186/1742-2094-9-180
[111] Kuang, X., Huang, Y., Gu, H. F., Zu, X. Y., Zou, W. Y., Song, Z. B., & Guo, Q. L. (2012). Effects of intrathecal epigallocatechin gallate, an inhibitor of Toll-like receptor 4, on chronic neuropathic pain in rats [Journal Article]. European Journal of Pharmacology, 676(1-3), 51-56. http://doi.org/10.1016/j.ejphar.2011.11.037
[112] Davaanyam, D., Lee, H., Seol, S. I., Oh, S. A., Kim, S. W., & Lee, J. K. (2023). HMGB1 induces hepcidin upregulation in astrocytes and causes an acute iron surge and subsequent ferroptosis in the postischemic brain [Journal Article; Research Support, Non-U.S. Gov't]. Experimental and Molecular Medicine, 55(11), 2402-2416. http://doi.org/10.1038/s12276-023-01111-z
[113] Liu, L., Dong, Y., Shan, X., Li, L., Xia, B., & Wang, H. (2019). Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo [Journal Article]. Molecules, 24(2) http://doi.org/10.3390/molecules24020326
[114] Franklin, T. C., Wohleb, E. S., Zhang, Y., Fogaca, M., Hare, B., & Duman, R. S. (2018). Persistent Increase in Microglial RAGE Contributes to Chronic Stress-Induced Priming of Depressive-like Behavior [Journal Article]. Biological Psychiatry, 83(1), 50-60. http://doi.org/10.1016/j.biopsych.2017.06.034
[115] Valdes-Ferrer, S. I., Rosas-Ballina, M., Olofsson, P. S., Lu, B., Dancho, M. E., Ochani, M., Li, J. H., Scheinerman, J. A., Katz, D. A., Levine, Y. A., Hudson, L. K., Yang, H., Pavlov, V. A., Roth, J., Blanc, L., Antoine, D. J., Chavan, S. S., Andersson, U., Diamond, B., & Tracey, K. J. (2013). HMGB1 mediates splenomegaly and expansion of splenic CD11b+ Ly-6C(high) inflammatory monocytes in murine sepsis survivors [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Journal of Internal Medicine, 274(4), 381-390. http://doi.org/10.1111/joim.12104
[116] Zhu, C., Chen, T., & Liu, B. (2018). Inhibitory effects of miR-25 targeting HMGB1 on macrophage secretion of inflammatory cytokines in sepsis [Journal Article]. Oncology Letters, 16(4), 5027-5033. http://doi.org/10.3892/ol.2018.9308
[117] Hagiwara, S., Iwasaka, H., Hasegawa, A., Koga, H., & Noguchi, T. (2008). Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin-induced acute lung injury in a rat model [Journal Article]. Critical Care Medicine, 36(8), 2407-2413. http://doi.org/10.1097/CCM.0b013e318180b3ba
[118] Chorny, A., & Delgado, M. (2008). Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1 [Journal Article; Research Support, Non-U.S. Gov't]. American Journal of Pathology, 172(5), 1297-1307. http://doi.org/10.2353/ajpath.2008.070969
[119] Yang, M., Cao, L., Xie, M., Yu, Y., Kang, R., Yang, L., Zhao, M., & Tang, D. (2013). Chloroquine inhibits HMGB1 inflammatory signaling and protects mice from lethal sepsis [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Biochemical Pharmacology, 86(3), 410-418. http://doi.org/10.1016/j.bcp.2013.05.013
[120] Wang, B., Koga, K., Osuga, Y., Hirata, T., Saito, A., Yoshino, O., Hirota, Y., Harada, M., Takemura, Y., Fujii, T., & Taketani, Y. (2011). High mobility group box 1 (HMGB1) levels in the placenta and in serum in preeclampsia [Journal Article; Research Support, Non-U.S. Gov't]. American Journal of Reproductive Immunology, 66(2), 143-148. http://doi.org/10.1111/j.1600-0897.2010.00975.x
[121] Li, W., Li, J., Ashok, M., Wu, R., Chen, D., Yang, L., Yang, H., Tracey, K. J., Wang, P., Sama, A. E., & Wang, H. (2007). A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1 [Journal Article; Research Support, N.I.H., Extramural]. Journal of Immunology, 178(6), 3856-3864. http://doi.org/10.4049/jimmunol.178.6.3856
[122] Nishiike, S., Hiramatsu, T., Shiraishi, M., Ueda, Y., & Tsuchida, H. (2013). Relationship between vascular reactivity and expression of HMGB1 in a rat model of septic aorta [Journal Article]. Journal of Anesthesia, 27(5), 684-692. http://doi.org/10.1007/s00540-013-1584-x
[123] Tang, D., Kang, R., Zeh, H. J., & Lotze, M. T. (2023). The multifunctional protein HMGB1: 50 years of discovery [Journal Article; Review]. Nature Reviews Immunology, 23(12), 824-841. http://doi.org/10.1038/s41577-023-00894-6
[124] Hou, W., Wei, X., Liang, J., Fang, P., Ma, C., Zhang, Q., & Gao, Y. (2021). HMGB1-Induced Hepatocyte Pyroptosis Expanding Inflammatory Responses Contributes to the Pathogenesis of Acute-on-Chronic Liver Failure (ACLF) [Journal Article]. Journal of Inflammation Research, 14, 7295-7313. http://doi.org/10.2147/JIR.S336626
[125] Jube, S., Rivera, Z. S., Bianchi, M. E., Powers, A., Wang, E., Pagano, I., Pass, H. I., Gaudino, G., Carbone, M., & Yang, H. (2012). Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cancer Research, 72(13), 3290-3301. http://doi.org/10.1158/0008-5472.CAN-11-3481
[126] Xue, J., Patergnani, S., Giorgi, C., Suarez, J., Goto, K., Bononi, A., Tanji, M., Novelli, F., Pastorino, S., Xu, R., Caroccia, N., Dogan, A. U., Pass, H. I., Tognon, M., Pinton, P., Gaudino, G., Mak, T. W., Carbone, M., & Yang, H. (2020). Asbestos induces mesothelial cell transformation via HMGB1-driven autophagy [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. Proceedings of the National Academy of Sciences of the United States of America, 117(41), 25543-25552. http://doi.org/10.1073/pnas.2007622117
[127] Mcdonald, K. A., Huang, H., Tohme, S., Loughran, P., Ferrero, K., Billiar, T., & Tsung, A. (2015). Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Molecular Medicine, 20(1), 639-648. http://doi.org/10.2119/molmed.2014.00076
[128] Chen, R., Huang, Y., Quan, J., Liu, J., Wang, H., Billiar, T. R., Lotze, M. T., Zeh, H. J., Kang, R., & Tang, D. (2020). HMGB1 as a potential biomarker and therapeutic target for severe COVID-19 [Journal Article]. Heliyon, 6(12), e05672. http://doi.org/10.1016/j.heliyon.2020.e05672
[129] Kumar S, N. R. M. V. (2020). Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Coronavirus Disease 2019 (COVID-19), 23-31.
[130] Li YG, W. X. L. Q. (2022). GLYCYRRHIZIN REGULATES HIPPOCAMPAL PATHOLOGICAL DAMAGE OF KAINIC ACID-INDUCED SEIZURES IN YOUNG RATS THROUGH HMGB1/TLR4/P-NF-KB PATHWAY. Acta Medica Mediterranea, 4(38), 2475-2479.
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Life Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2024-10-25
Accepted 2024-12-22
Published 2024-12-30


