Abstract
Ferroptosis is a new type of regulated cell death produced by iron-dependent accumulation of reactive oxygen species in lipids, which is different from apoptosis, pyroptosis and autophagy, and plays the role of a "double-edged sword" in tumor therapy. Ferroptosis-related lncRNA are involved in tumor cell death by regulating lipid metabolism and ferroptosis-associated genes, thus becoming one of the hotspots in the field of tumor-targeted therapy. In this paper, we review the role of ferroptosis and its related lncRNA in inhibiting the development of tumor cells and improving the therapeutic effect of drugs, which is expected to provide a new strategy for the treatment of tumors.
Keywords:Ferroptosis; Long non-coding RNAs; Oncotherapy
Introduction
The concept of "ferroptosis" was first introduced by Dixon et al. in 2012[1] . It represents a novel form of regulated cell death (RCD) that differs from apoptosis[2] and pyroptosis[3] . Ferroptosis is an iron-dependent mode of cell death characterized by the accumulation of reactive oxygen species (ROS) leading to lipid peroxidation (LPO)[4-6] . Consequently, similar to other forms of programmed cell death, ferroptosis is gradually emerging as a new therapeutic strategy in oncology. The application of ferroptosis in conjunction with small molecule drug therapies is expected to significantly improve cancer treatment methods.
Long non-coding RNAs (lncRNAs) are a class of RNA molecules longer than 200 nucleotides that do not encode proteins[7,8] . LncRNAs play crucial regulatory roles in various biological processes, influencing tumor cell proliferation, differentiation, metastasis, and apoptosis at multiple levels[9,10] . In tumor diagnosis and treatment, lncRNAs also represent promising biomarkers or therapeutic targets.[11,12] . Recent studies have confirmed that lncRNAs can regulate the occurrence of ferroptosis in tumor cells by mediating lipid metabolism and the expression of ferroptosis-related genes[13] . Thus, lncRNAs constitute an important area of research with broad application prospects, making them potential therapeutic agents for various tumors.
Current therapeutic options remain inadequate, with cancer mortality rates are rising each year. For instance, targeted drug therapy often leads to the development of drug resistance in tumor cells, significantly diminishing treatment efficacy and ultimately threatening patient safety[14] . Therefore, there is a pressing need to develop more effective targeted treatment strategies. Given the importance of lncRNAs and ferroptosis in cancer therapy, this paper reviews the molecular mechanisms of lncRNA-mediated ferroptosis in common clinical tumors and explores the therapeutic potential of ferroptosis-related lncRNAs, aiming to identify novel cancer treatment strategies.
The main mechanisms of lncRNA-mediated ferroptosis in cancer cells
Iron metabolism pathway mediating ferroptosis
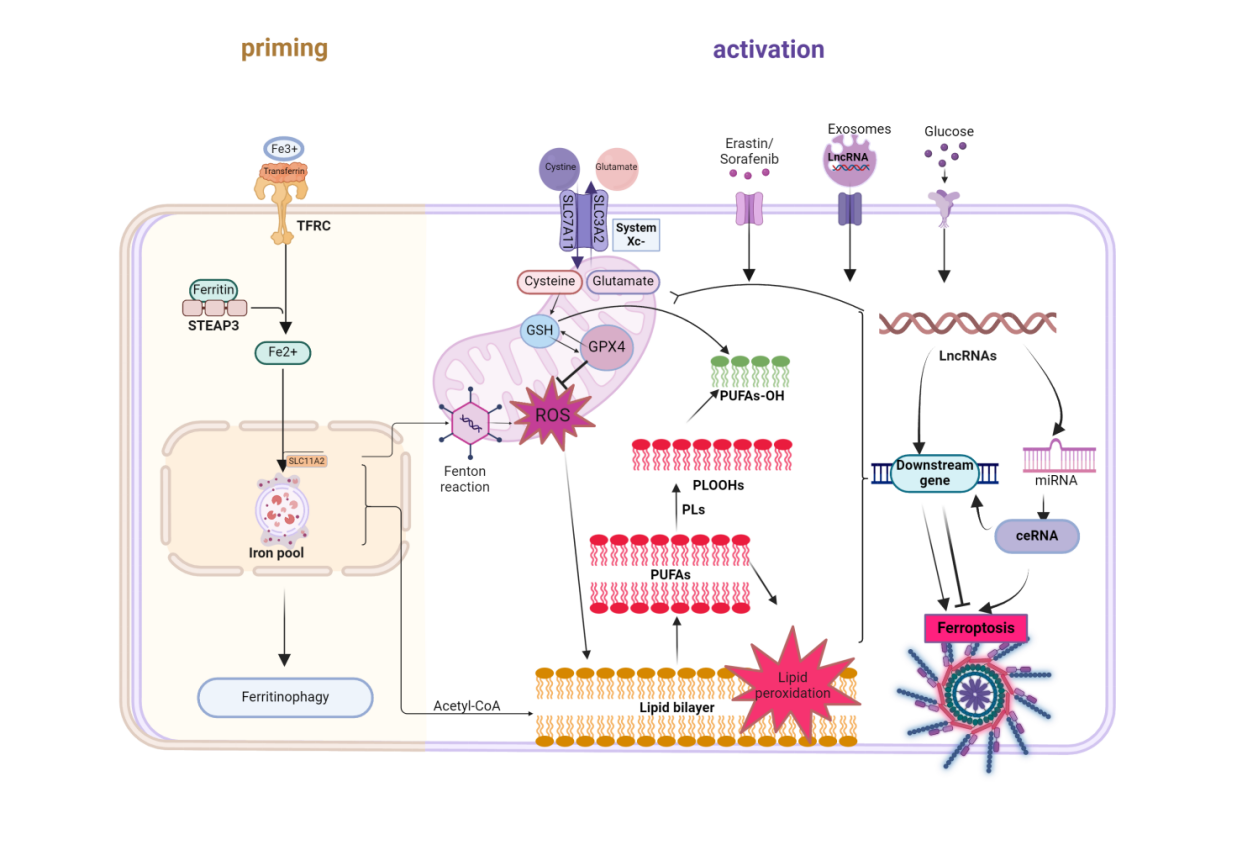
The molecular mechanisms underlying ferroptosis-induced tumor cell death are complex, involving multiple steps, genes, and proteins[15] . Iron ions participate in the execution of cellular ferroptosis through complex mechanisms[16] . Extracellular trivalent iron ions (Fe3+) bind to transferrin and are subsequently internalized into the cell through transferrin receptor1(TFR1). Subsequently, metalloreductase STEAP3 reduces Fe3+ to Fe2+, which is released into the cytoplasmic labile iron pool (LIP) via divalent metal-ion transporter 1 (DMT1)[6] ,[17] . The hydroxyl and lipid radicals in the iron pool can be catalyzed by iron ions, leading to the synthesis of polyunsaturated fatty acids (PUFAs) through the Fenton reaction[18,19] . When intracellular iron ions increase suddenly, Fenton reactions are likely to be significantly intensified, leading to excessive accumulation of lipid peroxides and resulting in cellular ferroptosis. Recent studies highlight the critical role of post-transcriptional regulation in iron metabolism for ferroptosis sensitivity. For example, hepcidin promotes intracellular Fe2+accumulation by degrading FPN1, thereby enhancing lipid peroxidation[20] .
However, the homeostasis of the iron pool is also tightly regulated by iron export proteins ferroportin(FPN1) and iron regulatory proteins (IRPs), thereby controlling intracellular free iron levels[21,22] . These findings underscore that iron ions act not merely as executors of ferroptosis but also as dynamically balanced elements through multi-layered protein networks that precisely determine cellular fate.
Lipid peroxidation pathway mediating ferroptosis
Lipid peroxidation is one of the most crucial causes of ferroptosis. The occurrence of lipid peroxidation in cells can lead to the destruction of the lipid bilayer and damage to organelles, ultimately resulting in cell rupture and death[23,24] . Abnormal lipid metabolism can provide the necessary material and energy for rapidly proliferating tumor cells, thus exacerbating their condition[9] . Studies have shown that metabolic stress can promote the binding of PUFAs to membrane phospholipids, making tumor cells more sensitive to ferroptosis[25] . PUFAs are typically enriched in cell membranes and organelle membranes and are prone to react with RO generated by Fe2+ and Fe2+-dependent enzymes, leading to ferroptosis[19,26] . The reaction between iron ions and lipid peroxidation can induce ferroptosis through the generation of ROS. On one hand, ROS can accumulate extensively through the Fenton reaction and inactivation of glutathione peroxidase 4 (GPX4). on the other hand, the continuously accumulating ROS can react with PUFAs, inducing the formation of LPO. Stockwell's research has found that iron chelators can inhibit the transfer of electrons from iron ions to oxygen, thereby reducing the production of ROS in tumor cells, further elucidating the relationship between iron ions and ferroptosis[27] .
The system Xc--GSH-GPX4 pathway mediating ferroptosis
The cystine-glutamate antiporter system (system Xc-) is a critical hub in regulating ferroptosis and serves as a key factor for cellular antioxidant defense[28,29] In the process of regulating ferroptosis, system Xc- can export intracellular glutamate to the extracellular environment in a 1:1 ratio while simultaneously importing extracellular cystine into the cell[30] . SLC7A11 is a multi-channel transmembrane protein that plays an essential role in the dimerization of system Xc-, mediating the reverse transport of cystine and glutamate[31] . This allows extracellular cystine to enter the membrane via the SLC7A11 protein, where it is oxidized to cysteine and subsequently catalyzed to produce glutathione (GSH), which participates in inhibiting tumor ferroptosis and the antioxidant process[32] . GSH is crucial for cellular antioxidant defense, and its depletion can lead to an accumulation of lipid ROS and damage to the cell membrane. Long-term deficiency of GSH may result in ferroptosis of the cell[33] .
GPX4 is an antioxidant enzyme that relies on glutathione as a co-reductant, catalyzing the conversion of harmful lipid peroxides into relatively harmless water or alcohol, thus promoting the stability of the cell membrane[34,35] . When GPX4 activity is impaired, its detoxifying effect on lipid peroxides is significantly reduced, leading to the occurrence of ferroptosis[36] . Saturated membrane lipids are insensitive to oxidative stress, while highly saturated membrane lipids help tumor cells avoid damage from ROS. When tumor cells express high levels of GPX4, they can reduce ROS production and maintain redox homeostasis, enabling them to escape ferroptosis.
Overall, the system Xc--GSH-GPX4 pathway plays a vital role in regulating ferroptosis in tumor cells. By increasing the activity of system Xc- and the synthesis of GSH, cells can enhance their antioxidant capacity and protect themselves from the harms of lipid peroxidation and ferroptosis. However, when the functions of system Xc- or GPX4 are compromised, cells become unable to effectively counter lipid peroxidation and ferroptosis, ultimately leading to cell death.
Other pathways mediating ferroptosis
Exosomes are vesicular substances secreted by various cells, ranging from approximately 40 to 100 nm in size[37] . They can transfer a variety of bioactive molecules, including proteins, mRNA, and lncRNA, making them a novel communicator in cell signaling. Studies have shown that lncRNA within exosomes can regulate the transport and storage of iron ions by interacting with ferritin. Ferritin is a protein capable of binding and stabilizing iron ions, allowing it to transport excess iron ions extracellularly, thereby reducing the accumulation of free iron within cells and preventing iron-induced oxidative stress and ferroptosis[38] . In addition, exosomes can influence the occurrence of ferroptosis by regulating the accumulation of ROS in cells. Exosomes secreted by tumor-associated fibroblasts have been found to inhibit ferroptosis in gastric cancer cells, one mechanism being the reduction of ROS accumulation, which protects cells from lipid peroxidation and other forms of damage. Consequently, lncRNA derived from exosomes presents a potential therapeutic strategy to modulate iron metabolism and counteract ferroptosis, thereby influencing disease progression. Further research into the mechanisms by which exosome-derived lncRNA regulate ferroptosis will contribute to the development of new treatment strategies and offer fresh perspectives for the therapy of related diseases.
In summary, ferroptosis-related lncRNA (Table 1) mediates the occurrence of ferroptosis in tumor cells through multiple pathways, including iron ion pathways, lipid peroxidation pathways, and the system Xc--GSH-GPX4 pathway. These pathways interact to coordinate the cellular responses to oxidative stress and lipid peroxidation, ultimately affecting the induction of ferroptosis.
Table 1 The role of ferroptosis and related lncRNAs in tumor
|
LncRNA Name |
Cancer Type |
Regulation |
Specific Molecular Mechanism |
Associated Drugs/Small Molecules |
|
KRAL |
HCC |
Antioxidant Pathway Regulation |
Acts as a ceRNA to sponge miR-141, upregulate Keap1, and inhibit the Nrf2-dependent antioxidant pathway |
5-Fluorouracil (5-FU) |
|
GABPB1-AS1 |
HCC |
Antioxidant Pathway Regulation |
Directly downregulates PRDX5, bypassing miRNA regulation, inducing ROS-mediated lipid peroxidation |
- |
|
PVT1 |
HCC |
System Xc--GPX4 Regulation |
Sponges miR-214-3p to relieve GPX4 suppression, leading to sorafenib resistance |
Ketamine (inhibits PVT1 activity) |
|
LINC01134 |
HCC |
System Xc--GPX4 Regulation |
Suppresses GPX4 expression via miRNA sponging |
Oxaliplatin |
|
NEAT1 |
HCC |
System Xc--GPX4 Regulation |
Acts as a ceRNA to sponge miR-362-3P, upregulates MIOX, and enhances NADPH/GSH production |
Erastin, RSL3 |
|
|
NSCLC |
p53-Independent Pathways |
Silencing reduces ACSL4, SLC7A11, and GPX4 expression, increasing Erastin sensitivity |
Erastin |
|
P53RRA |
NSCLC |
p53 -Dependent Regulatory |
Binds G3BP1 to enhance p53 nuclear retention, inhibiting SLC7A11 transcription |
- |
|
MEG3 |
NSCLC |
p53 -Dependent Regulatory Axis |
Upregulates p53 expression to suppress tumor proliferation |
Paclitaxel (PTX) |
|
LINC00336 |
NSCLC |
p53 -Dependent Regulatory Axis |
Interacts with miR6852 via the p53-LSH-ELAVL1 axis, activating the CBS pathway |
- |
|
FERO |
GC |
Lipid Metabolism Regulation |
Chemotherapy-induced upregulation stabilizes SCD1 mRNA via hnRNPA1, promoting MUFA synthesis to inhibit lipid peroxidation |
Cisplatin, Paclitaxel (induce FERO expression) |
|
ARHGEF26-AS1 |
EC |
Lipid Metabolism Regulation |
Sponges miR-372-3p to activate ADAM23, inhibiting GPX4, SLC3A2, and SLC7A11 |
- |
|
CBSLR |
GC |
Ubiquitination Regulation |
Recruits YTHDF2 under hypoxia to inhibit CBS mRNA transcription, reducing CBS expression and inducing ACSL4 ubiquitination |
- |
|
BDNF-AS |
GC |
Ubiquitination Regulation |
Activates VDAC3 ubiquitination via the BDNF-AS/WDR5/FBXW7 axis |
Erastin (targets VDAC3) |
|
OIP5-AS1 |
BCa |
System Xc--GPX4 Regulation |
1. Acts as ceRNA to sponge miR-128-3p, upregulating SLC7A11 (anti-ferroptosis) |
Cadmium (pro-ferroptosis) |
|
UCA1 |
BCa |
System Xc--GPX4 Regulation |
Sponges miR-16 to upregulate GLS2, driving glutamine metabolism and enhancing GSH synthesis/GPX4 activity |
- |
|
RP11-89 |
BCa |
Iron Metabolism Regulation |
Sponge miR-129-5p, upregulates PROM2, promoting exosomal iron efflux and reducing intracellular LIP |
- |
The Role of Ferroptosis and related lncRNAs in tumor progress
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the sixth most common cancer globally and the third leading cause of cancer-related deaths[39] . However, only a small number of HCC patients are diagnosed at an early stage, thus missing the optimal timing for surgical intervention[40] . Research has demonstrated that ferroptosis induction as a promising strategy, where multiple lncRNAs exhibit differential regulatory mechanisms through distinct molecular pathways[21] ,[41] . The current research reveals three predominant levels by which lncRNAs modulate ferroptosis in HCC:
Antioxidant Pathway Regulation
LncRNA and miRNA can regulate the occurrence of ferroptosis in HCC through the competing endogenous RNA (ceRNA) mechanism[42,43] . For instance, the lncRNA KRAL enhances the expression of Keap1 when bound to miR-141, leading to the inactivation of the Nrf2-dependent antioxidant pathway[44] . Nrf2 is an important antioxidant factor during oxidative stress responses and is regulated by Keap1 (Kelch ECH associating protein 1)[45] . Sun et al. found that knocking out Nrf2-related genes induces ferroptosis in cells, inhibiting the proliferation of HCC cells[46] . In the cytoplasm, Nrf2 regulates the expression and generation of intracellular antioxidant proteins[47] and ROS[48] by binding to antioxidant response elements (ARE). Additionally, ferroptosis-related lncRNA can directly regulate the expression of ROS in tumor cells, thereby mediating lipid peroxidation. For example, lncRNA GABPB1-AS1 directly downregulates PRDX5 to induce ROS accumulation, bypassing miRNA-mediated regulation. This mechanism delivers rapid oxidative damage but lacks tissue specificity, risking systemic oxidative stress[49] .
System Xc--GPX4 Regulation
For instance, when lncRNA PVT1 specifically binds to miR-214-3p, it reduces miR-214-3p inhibitory effect on GPX4, leading to an increase in GPX4 expression and promoting ferroptosis in liver cancer cells. Conversely, inhibiting miR-214-3p expression or overexpressing GPX4 can reverse ferroptosis[50] . Moreover, Kang et al. [51] found that lncRNA LINC01134 can promote ferroptosis by affecting GPX4 expression . The nuclear-enriched abundant transcript 1 (NEAT1) interacts with miR-362-3P through the ceRNA mechanism. promoting the expression of MIOX. Then, MIOX increases the production of NADPH and GSH. accelerating the occurrence of ferroptosis in HCC[52] .
Reprogramming of Drug Sensitivity
These ferroptosis-related lncRNAs may interact with chemotherapeutic agents or ferroptosis inducers, offering novel strategies to overcome treatment resistance in HCC. KRAL synergizes with 5-fluorouracil (5-FU) by activating the Nrf2-mediated antioxidant pathway. PVT1 can suppress the induction of ferroptosis in HCC by ketamine, resulting in drug resistance. LINC01134 specifically enhancing the sensitivity of liver cancer patients to oxaliplatin treatment. NEAT1 significantly increasing the efficacy of small-molecule drugs like Erastin and RSL3 in inducing ferroptosis. Cross-talk between lncRNAs (e.g., NEAT1 and LINC01134 co-targeting GPX4) may produce synergistic or antagonistic effects, necessitating systems biology approaches to construct regulatory network models.
These studies indicate that lncRNA holds great promise as a target for inducing ferroptosis in the therapeutic treatment of HCC. Collectively, ferroptosis-related lncRNAs redefine HCC treatment paradigms by targeting redox homeostasis and drug-response pathways, offering novel strategies to counteract therapeutic resistance.The current research is mainly based on in vitro cell models and in vivo animal models, which may not fully reflect the complex tumor microenvironment and heterogeneity in human patients. Future research should focus on validating the findings in larger clinical cohorts to better understand the translational potential of ferroptosis-related lncRNAs in HCC.
Lung cancer
Lung cancer is the second most common cancer worldwide and is also one of the leading causes of cancer-related deaths[39] . Current treatment options for Non-small cell lung cancer (NSCLC) include chemotherapy, radiotherapy, surgical treatment, and biological therapy[53] . Over the past two decades, most patients with advanced NSCLC develop resistance to treatment, leading to further disease progression[54] . Therefore, there is an urgent need to study personalized treatment regimens for lung cancer, particularly for NSCLC, and to develop rational and effective combination therapies[43,55] . Crucially, lncRNAs are emerging as key regulators of ferroptosis in NSCLC, primarily through their interaction with the p53 pathway and downstream metabolic networks. Below, we synthesize current findings by categorizing ferroptosis-related lncRNAs based on their functional mechanisms and clinical implications.
p53-Dependent Regulatory Axis
P53 gene, known as the "guardian of the genome", regulates cell survival and division under various stress conditions. Furthermore, p53 can induce ferroptosis in lung cancer by inhibiting SLC7A11[56] . Studies have found that lncRNA P53RRA promotes ferroptosis in lung cancer by binding to G3BP1 and enhancing p53 nuclear retention[57] . Additionally, the related genes were act in a negative regulatory manner with respect to p53[58] . For example, MEG3 can promote the expression of p53, thereby inhibiting NSCLC proliferation[59] . The combined use of lncRNA MEG3 and paclitaxel (PTX) significantly enhances the ability of PTX to kill tumor cells[60] . Additionnally, p53 may enhance the expression of lymphoid-specific helicase (LSH), which promotes ELAV-like RNA binding protein 1 (ELAVL1) expression and facilitates the interaction between LINC00336 and miR6852, ultimately activating cystathionine β-synthase (CBS), a marker of downstream ferroptosis-related pathways[61] .
p53-Independent Pathways
Certain lncRNAs bypass p53 to directly regulate ferroptosis effectors. Recently, Wu[62] conducted a more in-depth study on the mechanisms of ferroptosis-related lncRNA in NSCLC. They discovered that silencing lncRNA NEAT1, in conjunction with Erastin treatment, significantly reduced the expression of acyl-CoA synthetase long chain family member 4 (ACSL4), SLC7A11, and GPX4, thereby increasing tumor cell sensitivity to ferroptosis.
In summary, research on ferroptosis-related lncRNAs reveals that the p53 gene participates in regulating ferroptosis in lung cancer cells through multiple pathways. p53 mediates the occurrence of ferroptosis through the system Xc--GSH-GPX4 pathway, inhibiting the transcription of the SLC7A11 gene in the nucleus to reduce cystine uptake and thereby limiting intracellular glutathione production, ultimately leading to ferroptosis in NSCLC. However, the clinical sample sizes in these studies are often small, which restricts the statistical power and the ability to draw definitive conclusions. Larger, well-powered clinical trials are needed to validate these findings and to better understand the clinical relevance of lncRNA-mediated ferroptosis in NSCLC.
Gastric and Esophageal Cancers
Gastric cancer (GC) and esophageal cancer (EC) are major contributors to digestive system malignancies, characterized by high heterogeneity and limited therapeutic options[39] . Recent studies increasingly indicate that ferroptosis and lncRNAs play crucial roles in the regulation of gastric and esophageal cancers. Thus, it is essential to develop new therapeutic strategies based on ferroptosis-related lncRNAs for these malignancies.
Lipid Metabolism Regulation
Exosomes are pivotal in the initiation and progression of gastric cancer[63] . Zhang et al. [64] discovered that the chemotherapy-induced ferroptosis-related exosomal lncRNA FERO regulates ferroptosis in gastric cancer stem cells. Chemotherapy (cisplatin/paclitaxel) upregulates FERO via USP7, which recruits hnRNPA1 to stabilize SCD1 mRNA. SCD1 catalyzes MUFA synthesis, reducing lipid peroxidation and suppressing ferroptosis in GC stem cells[65] . In esophageal cancer research, Ren et al.[66] developed a system using ferroptosis and iron metabolism-related lncRNAs to predict survival in esophageal squamous cell carcinoma, emphasizing their relationship with the tumor development. In addition, lncRNA ARHGEF26-AS1 has been found to activate the expression of receptor ADAM23 by adsorbing miR-372-3p[67] . When both lncRNA ARHGEF26-AS1 and ADAM23 are underexpressed, the prognosis for esophageal cancer patients is generally poor. ADAM23 inhibits the protein levels of GPX4, SLC3A2, and SLC7A11, thereby promoting ferroptosis in esophageal cancer cells. Both in vivo and in vitro experiments demonstrate that overexpression of lncRNA ARHGEF26-AS1 and ADAM23 significantly suppresses tumor cell growth and metastasis.
Ubiquitination Regulation
Ubiquitination is involved in the regulation of ferroptosis in gastric cancer cells through epigenetic mechanisms[28] . Hypoxia-induced lncRNA CBSLR recruits YTHDF2 to the m6A-modified coding sequence of CBS mRNA, thereby inhibiting the transcription of CBS mRNA. The reduced expression level of CBS leads to a decrease in ACSL4 protein methylation, which in turn induces polyubiquitination and degradation of the corresponding protein, thus triggering ferroptosis GC[68,69] . Moreover, lncRNA BDNF-AS activates the ubiquitination of downstream VDAC3 via the BDNF-AS/WDR5/FBXW7 axis, influencing ferroptosis in GC[70] . VDAC3, a key gene for ion transport across mitochondria, is typically overexpressed in malignant tumors and serves as a target site for Erastin[71] . Clinically, enhancing the efficacy of anticancer drugs while promoting cellular ferroptosis may be achieved by upregulating VDAC3 expression through the BDNF-AS axis.
Research on ferroptosis-related lncRNAs provides insights into the mechanisms mediating ferroptosis from multiple perspectives, including exosomesand ubiquitination. This addresses the current limitations in ferroptosis treatment carriers[72] . Tumor-derived ferroptosis exosomal lncRNAs can communicate signals related to drug resistance and angiogenesis to neighboring cells, thereby promoting the proliferation of gastric cancer cells. Therefore, targeted therapy against lncRNA FERO in gastric cancer exosomes presents a promising exosome-based treatment strategy. Such studies hold promise for advancing clinical diagnosis and prognosis in digestive system tumors, potentially leading to more effective targeted therapies for these cancers.
Prostate and Bladder Cancers
Prostate cancer (PCa) and bladder cancer (BCa), the most prevalent malignancies in the urinary system, collectively account for significant global cancer burden[73] . Both cancers share critical clinical challenges including insufficient diagnostic biomarkers and therapeutic targets, highlighting the importance of investigating ferroptosis-related lncRNAs as potential therapeutic avenues[74-76] . This section integrates the roles played by lncRNA within the following two frameworks, emphasizing their clinical significance.
System Xc--GPX4 Regulation
Emerging evidence reveals lncRNAs modulate System Xc--GPX4 pathway through distinct mechanisms in urinary cancers. For instant, lncRNA OIP5-AS1 exhibits dual regulatory roles. First, OIP5-AS1 acts as a ceRNA by sequestering miR-128-3p, thereby upregulating SLC7A11 expression and suppressing ferroptosis. On the contrary, it can directly binds cadmium to downregulate SLC7A11, promoting cadmium-induced ferroptosis[77] . In addition, lncRNA UCA1 upregulates mitochondrial glutaminase 2 (GLS2) by sponging miR-16, driving glutamine metabolic reprogramming[78] . GLS2-mediated glutamine-to-glutamate conversion fuels system xc⁻- dependent cystine uptake and GSH synthesis, thereby suppressing lipid ROS toxicity[79] . Enhanced GSH sustains GPX4 activity to antagonize ferroptosis, while concurrently mitigating oxidative damage to promote bladder cancer survival[80] .
Iron Metabolism Regulation
LncRNA RP11-89 orchestrates ferroptosis resistance by rewiring iron homeostasis through exosome-mediated iron effluxin in bladder cancer[81] . RP11-89 functions as a ceRNA to sequester miR-129-5p, derepressing PROM2 expression. PROM2 encodes prominin2, a key driver of multivesicular body (MVB) biogenesis. Prominin2 facilitates the packaging of ferritin-bound iron into MVBs, which are subsequently secreted as exosomes, effectively depleting intracellular LIP and suppressing Fenton reaction-driven lipid peroxidation[82] .
In conclusion, it is evident that lncRNAs primarily modulate System Xc--GPX4 and iron metabolism within the urinary system, thereby mediating the onset of ferroptosis. Future targeted use of MVB and other novel carriers has the potential to considerably enhance the delivery of drugs or lncRNAs to target cells, thereby improving therapeutic outcomes. These carriers can surmount limitations associated with conventional drug delivery methods, such as poor drug solubility and stability. Targeting these pathways more specifically could augment the efficiency of ferroptosis in clinical applications.
Conclusion and future perspectives
Ferroptosis is a novel form of regulated cell death that is distinct from other forms such as autophagy and pyroptosis. Ferroptosis not only contributes to tumor cell death but is also linked to aging[83,84] and immunity[85,86] , providing new ideas and strategies for cancer immunotherapy and treatment. The relationship between ferroptosis and cancer therapy has a double-edged sword effect; however, our understanding of the relationship between lncRNA and ferroptosis remains limited. At present, the development of more precise animal models, such as patient-derived xenografts (PDX), will provide a better platform for studying ferroptosis and lncRNA responsiveness. Furthermore, integrating omics-based profiling, liquid biopsies, and machine learning models will be essential for overcoming challenges in ferroptosis research. Additionally, when combined with appropriate clinical chemotherapy agents, ferroptosis-related lncRNA can have a greater impact on cancer therapy, but the mechanisms involved and their clinical safety require further investigation.
Currently, some targeted drugs for caner, such as sorafenib, are widely used in the treatment of advanced HCC patients[87] . However, their effectiveness remains limited, while they can extend the overall survival of patients to some degree, many HCC patients have shown poor responses to sorafenib treatment and are prone to developing drug resistance[88,89] . On one hand, the use of sorafenib can result in significant side effects, burdening patients. On the other hand, its targeting mechanism for HCC is relatively singular, primarily functioning through the inhibition of tumor angiogenesis and targeting the Raf/MEK/ERK pathway, leading to limitations in targeted therapy. The related ferroptosis lncRNAs mainly induces ferroptosis in HCC by regulating GPX4, increasing lipid peroxidation and influencing the expression of other downstream ferroptosis-related iron metabolism genes. Moreover, they can significantly enhance tumor cells sensitivity to related small molecules, such as Erastin and commonly used clinical liver cancer drugs like sorafenib. Erastin specifically induces ferroptosis by inhibiting the system Xc--GSH-GPX4 pathway, increasing the feasibility of tumor-targeted therapies through combination with anticancer drugs. Although lncRNAs show promise in inducing ferroptosis, their clinical use has limitations: Firstly, the instability of lncRNAs can lead to low delivery efficiency, which is a major obstacle to their clinical use. Developing effective delivery systems (such as nanoparticles or inclusion bodies) is crucial for enhancing the stability and bioavailability of lncRNAs. Secondly, determining the optimal doses of lncRNA - based therapies and ferroptosis inducers is essential for maximizing therapeutic effects while minimizing side effects. This requires careful preclinical and clinical studies to establish dose - response relationships and identify the most effective combinations. Lastly, patients with different types of tumors may exhibit significant individual differences in their responses to combination therapies. Factors such as genetic background, tumor microenvironment, and previous treatment history may affect the effectiveness of different lncRNAs in mediating ferroptosis in different tumors. In conclusion, although the combination of lncRNA - mediated ferroptosis induction and traditional cancer therapies shows great potential, further research and development are needed to address clinical challenges and optimize treatment strategies. Future research should focus on improving the stability and delivery efficiency of lncRNAs, determining the optimal drug doses, understanding individual differences in treatment responses, and developing effective strategies to deal with potential adverse reactions.
Current research on lncRNAs mediating tumor ferroptosis remains limited, with their functional roles and mechanistic underpinnings incompletely characterized. In this review, we systematically analyze ferroptosis-associated lncRNAs across six malignancies spanning four biological systems, including hepatocellular carcinoma in the digestive system, and prostate/bladder cancers in the genitourinary system. We found that the mechanisms involved in ferroptosis vary across different tumors, but the core pathways are consistently centered around iron metabolism, lipid peroxidation, and the system Xc−- GPX4 pathway. Research on lncRNA - mediated ferroptosis in other types of tumors is also on the rise. In the future, the relationship between the two will be further supplemented, providing more help for clinical cancer treatment.
Acknowledgements
Figures in the article were drawnusing Biorender https://www.biorender.com/.
Author contributions
Wenpeng Pang was in charge of the proofreading and design of the manuscript. Qiulian Mo and Yunshan Qiu took responsibility for the writing of the manuscript. Yuni Liang, Qiaoying Wei, and Xuefei Fan were responsible for the collection and organization of literature on different tumors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Funding information
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
[1] DIXON SJ, LEMBERG KM, LAMPRECHT MR, SKOUTA R, ZAITSEV EM, GLEASON CE, et al. (2012).Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149(5): 1060-1072. https://doi.org/10.1016/j.cell.2012.03.042
[2] ELMORE S. (2007).Apoptosis: a review of programmed cell death. Toxicol Pathol, 35(4): 495-516. https://doi.org/10.1080/01926230701320337
[3] DU T, GAO J, LI P, WANG Y, QI Q, LIU X, et al. (2021).Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med, 11(8): e492. https://doi.org/10.1002/ctm2.492
[4] CAO JY, DIXON SJ. (2016).Mechanisms of ferroptosis. Cell Mol Life Sci, 73(11-12): 2195-2209. https://doi.org/10.1007/s00018-016-2194-1
[5] MOU Y, WANG J, WU J, HE D, ZHANG C, DUAN C, et al. (2019).Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol, 12(1): 34. https://doi.org/10.1186/s13045-019-0720-y
[6] XIE Y, HOU W, SONG X, YU Y, HUANG J, SUN X, et al. (2016).Ferroptosis: process and function. Cell Death Differ, 23(3): 369-379. https://doi.org/10.1038/cdd.2015.158
[7] CESANA M, CACCHIARELLI D, LEGNINI I, SANTINI T, STHANDIER O, CHINAPPI M, et al. (2011).A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell, 147(2): 358-369. https://doi.org/10.1016/j.cell.2011.09.028
[8] STATELLO L, GUO CJ, CHEN LL, HUARTE M. (2021).Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol, 22(2): 96-118. https://doi.org/10.1038/s41580-020-00315-9
[9] HUANG J, WANG J, HE H, HUANG Z, WU S, CHEN C, et al. (2021).Close interactions between lncRNAs, lipid metabolism and ferroptosis in cancer. Int J Biol Sci, 17(15): 4493-4513. https://doi.org/10.7150/ijbs.66181
[10] LIN W, ZHOU Q, WANG CQ, ZHU L, BI C, ZHANG S, et al. (2020).LncRNAs regulate metabolism in cancer. Int J Biol Sci, 16(7): 1194-1206. https://doi.org/10.7150/ijbs.40769
[11] LI J, MENG H, BAI Y, WANG K. (2016).Regulation of lncRNA and its role in cancer metastasis. Oncol Res, 23(5): 205-217. https://doi.org/10.3727/096504016x14549667334007
[12] XU W, ZHOU G, WANG H, LIU Y, CHEN B, CHEN W, et al. (2020).Circulating lncRNA SNHG11 as a novel biomarker for early diagnosis and prognosis of colorectal cancer. Int J Cancer, 146(10): 2901-2912. https://doi.org/10.1002/ijc.32747
[13] JIANG X, STOCKWELL BR, CONRAD M. (2021).Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol, 22(4): 266-282. https://doi.org/10.1038/s41580-020-00324-8
[14] VASAN N, BASELGA J, HYMAN DM. (2019).A view on drug resistance in cancer. Nature, 575(7782): 299-309. https://doi.org/10.1038/s41586-019-1730-1
[15] AVERETT C, BHARDWAJ A, ARORA S, SRIVASTAVA SK, KHAN MA, AHMAD A, et al. (2016).Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor-stromal cross-talk. Carcinogenesis, 37(11): 1052-1061. https://doi.org/10.1093/carcin/bgw096
[16] TANG D, KROEMER G. (2020).Ferroptosis. Curr Biol, 30(21): R1292-r1297. https://doi.org/10.1016/j.cub.2020.09.068
[17] GRAHAM RM, CHUA AC, HERBISON CE, OLYNYK JK, TRINDER D. (2007).Liver iron transport. World J Gastroenterol, 13(35): 4725-4736. https://doi.org/10.3748/wjg.v13.i35.4725
[18] HE YJ, LIU XY, XING L, WAN X, CHANG X, JIANG HL. (2020).Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials, 241: 119911. https://doi.org/10.1016/j.biomaterials.2020.119911
[19] CHENG Z, LI Y. (2007).What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem Rev, 107(3): 748-766. https://doi.org/10.1021/cr040077w
[20] BAO X, LUO X, BAI X, LV Y, WENG X, ZHANG S, et al. (2023).Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic Biol Med, 201: 76-88. https://doi.org/10.1016/j.freeradbiomed.2023.03.006
[21] WILBON AS, SHEN J, RUCHALA P, ZHOU M, PAN Y. (2023).Structural basis of ferroportin inhibition by minihepcidin PR73. PLoS Biol, 21(1): e3001936. https://doi.org/10.1371/journal.pbio.3001936
[22] CARDONA CJ, MONTGOMERY MR. (2023).Iron regulatory proteins: players or pawns in ferroptosis and cancer? Front Mol Biosci, 10: 1229710. https://doi.org/10.3389/fmolb.2023.1229710
[23] VON KRUSENSTIERN AN, ROBSON RN, QIAN N, QIU B, HU F, REZNIK E, et al. (2023).Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol, 19(6): 719-730. https://doi.org/10.1038/s41589-022-01249-3
[24] LIANG D, MINIKES AM, JIANG X. (2022).Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell, 82(12): 2215-2227. https://doi.org/10.1016/j.molcel.2022.03.022
[25] YUAN ZH, LIU T, WANG H, XUE LX, WANG JJ. (2021).Fatty Acids Metabolism: The Bridge Between Ferroptosis and Ionizing Radiation. Front Cell Dev Biol, 9: 675617. https://doi.org/10.3389/fcell.2021.675617
[26] YANG WS, STOCKWELL BR. (2016).Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol, 26(3): 165-176. https://doi.org/10.1016/j.tcb.2015.10.014
[27] DIXON SJ, STOCKWELL BR. (2014).The role of iron and reactive oxygen species in cell death. Nat Chem Biol, 10(1): 9-17. https://doi.org/10.1038/nchembio.1416
[28] CHEN X, LI J, KANG R, KLIONSKY DJ, TANG D. (2021).Ferroptosis: machinery and regulation. Autophagy, 17(9): 2054-2081. https://doi.org/10.1080/15548627.2020.1810918
[29] DE BAAT A, MEIER DT, FONTANA A, BöNI-SCHNETZLER M, DONATH MY. (2023).Cystine/Glutamate antiporter system xc- deficiency impairs macrophage glutathione metabolism and cytokine production. PLoS One, 18(10): e0291950. https://doi.org/10.1371/journal.pone.0291950
[30] ZHAO Y, LI Y, ZHANG R, WANG F, WANG T, JIAO Y. (2020).The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther, 13: 5429-5441. https://doi.org/10.2147/ott.S254995
[31] YAN Y, TENG H, HANG Q, KONDIPARTHI L, LEI G, HORBATH A, et al. (2023).SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun, 14(1): 3673. https://doi.org/10.1038/s41467-023-39401-9
[32] KOPPULA P, ZHUANG L, GAN B. (2021).Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell, 12(8): 599-620. https://doi.org/10.1007/s13238-020-00789-5
[33] XU T, DING W, JI X, AO X, LIU Y, YU W, et al. (2019).Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med, 23(8): 4900-4912. https://doi.org/10.1111/jcmm.14511
[34] LATUNDE-DADA GO. (2017).Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj, 1861(8): 1893-1900. https://doi.org/10.1016/j.bbagen.2017.05.019
[35] URSINI F, MAIORINO M. (2020).Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med, 152: 175-185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
[36] SEILER A, SCHNEIDER M, FöRSTER H, ROTH S, WIRTH EK, CULMSEE C, et al. (2008).Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab, 8(3): 237-248. https://doi.org/10.1016/j.cmet.2008.07.005
[37] THéRY C, ZITVOGEL L, AMIGORENA S. (2002).Exosomes: composition, biogenesis and function. Nat Rev Immunol, 2(8): 569-579. https://doi.org/10.1038/nri855
[38] BROWN CW, MERCURIO AM. (2020).Ferroptosis resistance mediated by exosomal release of iron. Mol Cell Oncol, 7(3): 1730144. https://doi.org/10.1080/23723556.2020.1730144
[39] SUNG H, FERLAY J, SIEGEL RL, LAVERSANNE M, SOERJOMATARAM I, JEMAL A, et al. (2021).Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 71(3): 209-249. https://doi.org/10.3322/caac.21660
[40] NIE J, LIN B, ZHOU M, WU L, ZHENG T. (2018).Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol, 144(12): 2329-2337. https://doi.org/10.1007/s00432-018-2740-3
[41] CAPELLETTI MM, MANCEAU H, PUY H, PEOC'H K. (2020).Ferroptosis in liver diseases: an overview. Int J Mol Sci, 21(14): 4908. https://doi.org/10.3390/ijms21144908
[42] QIN Y, ZHANG D, ZHANG H, HOU L, WANG Z, YANG L, et al. (2022).Construction of a ferroptosis-related five-lncRNA signature for predicting prognosis and immune response in thyroid carcinoma. Cancer Cell Int, 22(1): 296. https://doi.org/10.1186/s12935-022-02674-z
[43] FEI X, HU C, WANG X, LU C, CHEN H, SUN B, et al. (2021).Construction of a Ferroptosis-Related Long Non-coding RNA Prognostic Signature and Competing Endogenous RNA Network in Lung Adenocarcinoma. Front Cell Dev Biol, 9: 751490. https://doi.org/10.3389/fcell.2021.751490
[44] WU L, PAN C, WEI X, SHI Y, ZHENG J, LIN X, et al. (2018).lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun Signal, 16(1): 47. https://doi.org/10.1186/s12964-018-0260-z
[45] NGUYEN T, NIOI P, PICKETT CB. (2009).The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem, 284(20): 13291-13295. https://doi.org/10.1074/jbc.R900010200
[46] SUN X, OU Z, CHEN R, NIU X, CHEN D, KANG R, et al. (2016).Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology, 63(1): 173-184. https://doi.org/10.1002/hep.28251
[47] KANSANEN E, KUOSMANEN SM, LEINONEN H, LEVONEN AL. (2013).The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol, 1(1): 45-49. https://doi.org/10.1016/j.redox.2012.10.001
[48] DENICOLA GM, KARRETH FA, HUMPTON TJ, GOPINATHAN A, WEI C, FRESE K, et al. (2011).Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354): 106-109. https://doi.org/10.1038/nature10189
[49] QI W, LI Z, XIA L, DAI J, ZHANG Q, WU C, et al. (2019).LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep, 9(1): 16185. https://doi.org/10.1038/s41598-019-52837-8
[50] HE GN, BAO NR, WANG S, XI M, ZHANG TH, CHEN FS. (2021).Ketamine Induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther, 15: 3965-3978. https://doi.org/10.2147/dddt.S332847
[51] KANG X, HUO Y, JIA S, HE F, LI H, ZHOU Q, et al. (2022).Silenced LINC01134 enhances oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. Front Oncol, 12: 939605. https://doi.org/10.3389/fonc.2022.939605
[52] ZHANG Y, LUO M, CUI X, O'CONNELL D, YANG Y. (2022).Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ, 29(9): 1850-1863. https://doi.org/10.1038/s41418-022-00970-9
[53] ALDUAIS Y, ZHANG H, FAN F, CHEN J, CHEN B. (2023).Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine (Baltimore), 102(8): e32899. https://doi.org/10.1097/md.0000000000032899
[54] RAMALINGAM SS, VANSTEENKISTE J, PLANCHARD D, CHO BC, GRAY JE, OHE Y, et al. (2020).Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med, 382(1): 41-50. https://doi.org/10.1056/NEJMoa1913662
[55] WANG M, HERBST RS, BOSHOFF C. (2021).Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med, 27(8): 1345-1356. https://doi.org/10.1038/s41591-021-01450-2
[56] KANG R, KROEMER G, TANG D. (2019).The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med, 133: 162-168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
[57] MAO C, WANG X, LIU Y, WANG M, YAN B, JIANG Y, et al. (2018).A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res, 78(13): 3484-3496. https://doi.org/10.1158/0008-5472.Can-17-3454
[58] ZHANG EB, YIN DD, SUN M, KONG R, LIU XH, YOU LH, et al. (2014).P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis, 5(5): e1243. https://doi.org/10.1038/cddis.2014.201
[59] LU KH, LI W, LIU XH, SUN M, ZHANG ML, WU WQ, et al. (2013).Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer, 13: 461. https://doi.org/10.1186/1471-2407-13-461
[60] XU J, SU C, ZHAO F, TAO J, HU D, SHI A, et al. (2018).Paclitaxel promotes lung cancer cell apoptosis via MEG3-P53 pathway activation. Biochem Biophys Res Commun, 504(1): 123-128. https://doi.org/10.1016/j.bbrc.2018.08.142
[61] HAYANO M, YANG WS, CORN CK, PAGANO NC, STOCKWELL BR. (2016).Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ, 23(2): 270-278. https://doi.org/10.1038/cdd.2015.93
[62] WU H, LIU A. (2021).Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J Int Med Res, 49(3): 300060521996183. https://doi.org/10.1177/0300060521996183
[63] FU M, GU J, JIANG P, QIAN H, XU W, ZHANG X. (2019).Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer, 18(1): 41. https://doi.org/10.1186/s12943-019-1001-7
[64] ZHANG H, WANG M, HE Y, DENG T, LIU R, WANG W, et al. (2021).Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis, 12(12): 1116. https://doi.org/10.1038/s41419-021-04406-z
[65] PéREZ-HERAS AM, MAYNERIS-PERXACHS J, COFáN M, SERRA-MIR M, CASTELLOTE AI, LóPEZ-SABATER C, et al. (2018).Long-chain n-3 PUFA supplied by the usual diet decrease plasma stearoyl-CoA desaturase index in non-hypertriglyceridemic older adults at high vascular risk. Clin Nutr, 37(1): 157-162. https://doi.org/10.1016/j.clnu.2016.11.009
[66] NIU R, ZHAO F, DONG Z, LI Z, LI S. (2022).A stratification system of ferroptosis and iron-metabolism related LncRNAs guides the prediction of the survival of patients with esophageal squamous cell carcinoma. Front Oncol, 12: 1010074. https://doi.org/10.3389/fonc.2022.1010074
[67] CHEN C, ZHAO J, LIU JN, SUN C. (2021).Mechanism and role of the neuropeptide LGI1 receptor ADAM23 in regulating biomarkers of ferroptosis and progression of esophageal cancer. Dis Markers, 2021: 9227897. https://doi.org/10.1155/2021/9227897
[68] YANG H, HU Y, WENG M, LIU X, WAN P, HU Y, et al. (2022).Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J Adv Res, 37: 91-106. https://doi.org/10.1016/j.jare.2021.10.001
[69] YAO X, YANG P, JIN Z, JIANG Q, GUO R, XIE R, et al. (2019).Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials, 197: 268-283. https://doi.org/10.1016/j.biomaterials.2019.01.026
[70] HUANG G, XIANG Z, WU H, HE Q, DOU R, LIN Z, et al. (2022).The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int J Biol Sci, 18(4): 1415-1433. https://doi.org/10.7150/ijbs.69454
[71] MALDONADO EN, SHELDON KL, DEHART DN, PATNAIK J, MANEVICH Y, TOWNSEND DM, et al. (2013).Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem, 288(17): 11920-11929. https://doi.org/10.1074/jbc.M112.433847
[72] PANG JY, LI PL, AFERIN B, YAN Y, WANG JM. (2021). New strategies for cancer treatment based on ferroptosis. Modern Oncology, 29(06): 1058-1061.
[73] BRAY F, LAVERSANNE M, SUNG H, FERLAY J, SIEGEL RL, SOERJOMATARAM I, et al. (2024).Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 74(3): 229-263. https://doi.org/10.3322/caac.21834
[74] CASADEI C, DIZMAN N, SCHEPISI G, CURSANO MC, BASSO U, SANTINI D, et al. (2019).Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol, 11: 1758835919890285. https://doi.org/10.1177/1758835919890285
[75] HERNANDO POLO S, MORENO MUñOZ D, ROSERO RODRíGUEZ AC, SILVA RUIZ J, ROSERO RODRíGUEZ DI, COUñAGO F. (2021).Changing the History of Prostate Cancer with New Targeted Therapies. Biomedicines, 9(4):392. https://doi.org/10.3390/biomedicines9040392
[76] POSADAS EM, LIMVORASAK S, FIGLIN RA. (2017).Targeted therapies for renal cell carcinoma. Nat Rev Nephrol, 13(8): 496-511. https://doi.org/10.1038/nrneph.2017.82
[77] ZHANG Y, GUO S, WANG S, LI X, HOU D, LI H, et al. (2021).LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf, 220: 112376. https://doi.org/10.1016/j.ecoenv.2021.112376
[78] LI HJ, LI X, PANG H, PAN JJ, XIE XJ, CHEN W. (2015).Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer. Jpn J Clin Oncol, 45(11): 1055-1063. https://doi.org/10.1093/jjco/hyv132
[79] BUCZKOWSKA J, SZELIGA M. (2023).Two Faces of Glutaminase GLS2 in Carcinogenesis. Cancers (Basel), 15(23). https://doi.org/10.3390/cancers15235566
[80] JIAO K, CHENG J, WANG Q, HAO M. (2024).LncRNA UCA1 enhances NRF2 expression through the m(6)A pathway to mitigate oxidative stress and ferroptosis in aging cardiomyocytes. J Bioenerg Biomembr, 56(6): 607-617. https://doi.org/10.1007/s10863-024-10045-8
[81] LUO W, WANG J, XU W, MA C, WAN F, HUANG Y, et al. (2021).LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis, 12(11): 1043. https://doi.org/10.1038/s41419-021-04296-1
[82] BROWN CW, AMANTE JJ, CHHOY P, ELAIMY AL, LIU H, ZHU LJ, et al. (2019).Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell, 51(5): 575-586.e574. https://doi.org/10.1016/j.devcel.2019.10.007
[83] CORADDUZZA D, CONGIARGIU A, CHEN Z, ZINELLU A, CARRU C, MEDICI S. (2023).Ferroptosis and Senescence: A Systematic Review. Int J Mol Sci, 24(4). https://doi.org/10.3390/ijms24043658
[84] MAZHAR M, DIN AU, ALI H, YANG G, REN W, WANG L, et al. (2021).Implication of ferroptosis in aging. Cell Death Discov, 7(1): 149. https://doi.org/10.1038/s41420-021-00553-6
[85] GONG D, CHEN M, WANG Y, SHI J, HOU Y. (2022).Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discov, 8(1): 427. https://doi.org/10.1038/s41420-022-01218-8
[86] LU Y, XIE X, LUO L. (2024).Ferroptosis crosstalk in anti-tumor immunotherapy: molecular mechanisms, tumor microenvironment, application prospects. Apoptosis, 29(11-12): 1914-1943. https://doi.org/10.1007/s10495-024-01997-8
[87] LI Q, CHEN K, ZHANG T, JIANG D, CHEN L, JIANG J, et al. (2023).Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur J Pharmacol, 955: 175913. https://doi.org/10.1016/j.ejphar.2023.175913
[88] PEARSON H, MARSHALL LV, CARCELLER F. (2020).Sorafenib in pediatric hepatocellular carcinoma from a clinician perspective. Pediatr Hematol Oncol, 37(5): 412-423. https://doi.org/10.1080/08880018.2020.1740844
[89] LADD AD, DUARTE S, SAHIN I, ZARRINPAR A. (2024).Mechanisms of drug resistance in HCC. Hepatology, 79(4): 926-940. https://doi.org/10.1097/hep.0000000000000237
 Figures
Figures References
References Peer
Peer Information
Information[1] DIXON SJ, LEMBERG KM, LAMPRECHT MR, SKOUTA R, ZAITSEV EM, GLEASON CE, et al. (2012).Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149(5): 1060-1072. https://doi.org/10.1016/j.cell.2012.03.042
[2] ELMORE S. (2007).Apoptosis: a review of programmed cell death. Toxicol Pathol, 35(4): 495-516. https://doi.org/10.1080/01926230701320337
[3] DU T, GAO J, LI P, WANG Y, QI Q, LIU X, et al. (2021).Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med, 11(8): e492. https://doi.org/10.1002/ctm2.492
[4] CAO JY, DIXON SJ. (2016).Mechanisms of ferroptosis. Cell Mol Life Sci, 73(11-12): 2195-2209. https://doi.org/10.1007/s00018-016-2194-1
[5] MOU Y, WANG J, WU J, HE D, ZHANG C, DUAN C, et al. (2019).Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol, 12(1): 34. https://doi.org/10.1186/s13045-019-0720-y
[6] XIE Y, HOU W, SONG X, YU Y, HUANG J, SUN X, et al. (2016).Ferroptosis: process and function. Cell Death Differ, 23(3): 369-379. https://doi.org/10.1038/cdd.2015.158
[7] CESANA M, CACCHIARELLI D, LEGNINI I, SANTINI T, STHANDIER O, CHINAPPI M, et al. (2011).A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell, 147(2): 358-369. https://doi.org/10.1016/j.cell.2011.09.028
[8] STATELLO L, GUO CJ, CHEN LL, HUARTE M. (2021).Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol, 22(2): 96-118. https://doi.org/10.1038/s41580-020-00315-9
[9] HUANG J, WANG J, HE H, HUANG Z, WU S, CHEN C, et al. (2021).Close interactions between lncRNAs, lipid metabolism and ferroptosis in cancer. Int J Biol Sci, 17(15): 4493-4513. https://doi.org/10.7150/ijbs.66181
[10] LIN W, ZHOU Q, WANG CQ, ZHU L, BI C, ZHANG S, et al. (2020).LncRNAs regulate metabolism in cancer. Int J Biol Sci, 16(7): 1194-1206. https://doi.org/10.7150/ijbs.40769
[11] LI J, MENG H, BAI Y, WANG K. (2016).Regulation of lncRNA and its role in cancer metastasis. Oncol Res, 23(5): 205-217. https://doi.org/10.3727/096504016x14549667334007
[12] XU W, ZHOU G, WANG H, LIU Y, CHEN B, CHEN W, et al. (2020).Circulating lncRNA SNHG11 as a novel biomarker for early diagnosis and prognosis of colorectal cancer. Int J Cancer, 146(10): 2901-2912. https://doi.org/10.1002/ijc.32747
[13] JIANG X, STOCKWELL BR, CONRAD M. (2021).Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol, 22(4): 266-282. https://doi.org/10.1038/s41580-020-00324-8
[14] VASAN N, BASELGA J, HYMAN DM. (2019).A view on drug resistance in cancer. Nature, 575(7782): 299-309. https://doi.org/10.1038/s41586-019-1730-1
[15] AVERETT C, BHARDWAJ A, ARORA S, SRIVASTAVA SK, KHAN MA, AHMAD A, et al. (2016).Honokiol suppresses pancreatic tumor growth, metastasis and desmoplasia by interfering with tumor-stromal cross-talk. Carcinogenesis, 37(11): 1052-1061. https://doi.org/10.1093/carcin/bgw096
[16] TANG D, KROEMER G. (2020).Ferroptosis. Curr Biol, 30(21): R1292-r1297. https://doi.org/10.1016/j.cub.2020.09.068
[17] GRAHAM RM, CHUA AC, HERBISON CE, OLYNYK JK, TRINDER D. (2007).Liver iron transport. World J Gastroenterol, 13(35): 4725-4736. https://doi.org/10.3748/wjg.v13.i35.4725
[18] HE YJ, LIU XY, XING L, WAN X, CHANG X, JIANG HL. (2020).Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials, 241: 119911. https://doi.org/10.1016/j.biomaterials.2020.119911
[19] CHENG Z, LI Y. (2007).What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: an update. Chem Rev, 107(3): 748-766. https://doi.org/10.1021/cr040077w
[20] BAO X, LUO X, BAI X, LV Y, WENG X, ZHANG S, et al. (2023).Cigarette tar mediates macrophage ferroptosis in atherosclerosis through the hepcidin/FPN/SLC7A11 signaling pathway. Free Radic Biol Med, 201: 76-88. https://doi.org/10.1016/j.freeradbiomed.2023.03.006
[21] WILBON AS, SHEN J, RUCHALA P, ZHOU M, PAN Y. (2023).Structural basis of ferroportin inhibition by minihepcidin PR73. PLoS Biol, 21(1): e3001936. https://doi.org/10.1371/journal.pbio.3001936
[22] CARDONA CJ, MONTGOMERY MR. (2023).Iron regulatory proteins: players or pawns in ferroptosis and cancer? Front Mol Biosci, 10: 1229710. https://doi.org/10.3389/fmolb.2023.1229710
[23] VON KRUSENSTIERN AN, ROBSON RN, QIAN N, QIU B, HU F, REZNIK E, et al. (2023).Identification of essential sites of lipid peroxidation in ferroptosis. Nat Chem Biol, 19(6): 719-730. https://doi.org/10.1038/s41589-022-01249-3
[24] LIANG D, MINIKES AM, JIANG X. (2022).Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell, 82(12): 2215-2227. https://doi.org/10.1016/j.molcel.2022.03.022
[25] YUAN ZH, LIU T, WANG H, XUE LX, WANG JJ. (2021).Fatty Acids Metabolism: The Bridge Between Ferroptosis and Ionizing Radiation. Front Cell Dev Biol, 9: 675617. https://doi.org/10.3389/fcell.2021.675617
[26] YANG WS, STOCKWELL BR. (2016).Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol, 26(3): 165-176. https://doi.org/10.1016/j.tcb.2015.10.014
[27] DIXON SJ, STOCKWELL BR. (2014).The role of iron and reactive oxygen species in cell death. Nat Chem Biol, 10(1): 9-17. https://doi.org/10.1038/nchembio.1416
[28] CHEN X, LI J, KANG R, KLIONSKY DJ, TANG D. (2021).Ferroptosis: machinery and regulation. Autophagy, 17(9): 2054-2081. https://doi.org/10.1080/15548627.2020.1810918
[29] DE BAAT A, MEIER DT, FONTANA A, BöNI-SCHNETZLER M, DONATH MY. (2023).Cystine/Glutamate antiporter system xc- deficiency impairs macrophage glutathione metabolism and cytokine production. PLoS One, 18(10): e0291950. https://doi.org/10.1371/journal.pone.0291950
[30] ZHAO Y, LI Y, ZHANG R, WANG F, WANG T, JIAO Y. (2020).The role of erastin in ferroptosis and its prospects in cancer therapy. Onco Targets Ther, 13: 5429-5441. https://doi.org/10.2147/ott.S254995
[31] YAN Y, TENG H, HANG Q, KONDIPARTHI L, LEI G, HORBATH A, et al. (2023).SLC7A11 expression level dictates differential responses to oxidative stress in cancer cells. Nat Commun, 14(1): 3673. https://doi.org/10.1038/s41467-023-39401-9
[32] KOPPULA P, ZHUANG L, GAN B. (2021).Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell, 12(8): 599-620. https://doi.org/10.1007/s13238-020-00789-5
[33] XU T, DING W, JI X, AO X, LIU Y, YU W, et al. (2019).Molecular mechanisms of ferroptosis and its role in cancer therapy. J Cell Mol Med, 23(8): 4900-4912. https://doi.org/10.1111/jcmm.14511
[34] LATUNDE-DADA GO. (2017).Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj, 1861(8): 1893-1900. https://doi.org/10.1016/j.bbagen.2017.05.019
[35] URSINI F, MAIORINO M. (2020).Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med, 152: 175-185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
[36] SEILER A, SCHNEIDER M, FöRSTER H, ROTH S, WIRTH EK, CULMSEE C, et al. (2008).Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab, 8(3): 237-248. https://doi.org/10.1016/j.cmet.2008.07.005
[37] THéRY C, ZITVOGEL L, AMIGORENA S. (2002).Exosomes: composition, biogenesis and function. Nat Rev Immunol, 2(8): 569-579. https://doi.org/10.1038/nri855
[38] BROWN CW, MERCURIO AM. (2020).Ferroptosis resistance mediated by exosomal release of iron. Mol Cell Oncol, 7(3): 1730144. https://doi.org/10.1080/23723556.2020.1730144
[39] SUNG H, FERLAY J, SIEGEL RL, LAVERSANNE M, SOERJOMATARAM I, JEMAL A, et al. (2021).Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 71(3): 209-249. https://doi.org/10.3322/caac.21660
[40] NIE J, LIN B, ZHOU M, WU L, ZHENG T. (2018).Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol, 144(12): 2329-2337. https://doi.org/10.1007/s00432-018-2740-3
[41] CAPELLETTI MM, MANCEAU H, PUY H, PEOC'H K. (2020).Ferroptosis in liver diseases: an overview. Int J Mol Sci, 21(14): 4908. https://doi.org/10.3390/ijms21144908
[42] QIN Y, ZHANG D, ZHANG H, HOU L, WANG Z, YANG L, et al. (2022).Construction of a ferroptosis-related five-lncRNA signature for predicting prognosis and immune response in thyroid carcinoma. Cancer Cell Int, 22(1): 296. https://doi.org/10.1186/s12935-022-02674-z
[43] FEI X, HU C, WANG X, LU C, CHEN H, SUN B, et al. (2021).Construction of a Ferroptosis-Related Long Non-coding RNA Prognostic Signature and Competing Endogenous RNA Network in Lung Adenocarcinoma. Front Cell Dev Biol, 9: 751490. https://doi.org/10.3389/fcell.2021.751490
[44] WU L, PAN C, WEI X, SHI Y, ZHENG J, LIN X, et al. (2018).lncRNA KRAL reverses 5-fluorouracil resistance in hepatocellular carcinoma cells by acting as a ceRNA against miR-141. Cell Commun Signal, 16(1): 47. https://doi.org/10.1186/s12964-018-0260-z
[45] NGUYEN T, NIOI P, PICKETT CB. (2009).The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem, 284(20): 13291-13295. https://doi.org/10.1074/jbc.R900010200
[46] SUN X, OU Z, CHEN R, NIU X, CHEN D, KANG R, et al. (2016).Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology, 63(1): 173-184. https://doi.org/10.1002/hep.28251
[47] KANSANEN E, KUOSMANEN SM, LEINONEN H, LEVONEN AL. (2013).The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol, 1(1): 45-49. https://doi.org/10.1016/j.redox.2012.10.001
[48] DENICOLA GM, KARRETH FA, HUMPTON TJ, GOPINATHAN A, WEI C, FRESE K, et al. (2011).Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature, 475(7354): 106-109. https://doi.org/10.1038/nature10189
[49] QI W, LI Z, XIA L, DAI J, ZHANG Q, WU C, et al. (2019).LncRNA GABPB1-AS1 and GABPB1 regulate oxidative stress during erastin-induced ferroptosis in HepG2 hepatocellular carcinoma cells. Sci Rep, 9(1): 16185. https://doi.org/10.1038/s41598-019-52837-8
[50] HE GN, BAO NR, WANG S, XI M, ZHANG TH, CHEN FS. (2021).Ketamine Induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther, 15: 3965-3978. https://doi.org/10.2147/dddt.S332847
[51] KANG X, HUO Y, JIA S, HE F, LI H, ZHOU Q, et al. (2022).Silenced LINC01134 enhances oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. Front Oncol, 12: 939605. https://doi.org/10.3389/fonc.2022.939605
[52] ZHANG Y, LUO M, CUI X, O'CONNELL D, YANG Y. (2022).Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ, 29(9): 1850-1863. https://doi.org/10.1038/s41418-022-00970-9
[53] ALDUAIS Y, ZHANG H, FAN F, CHEN J, CHEN B. (2023).Non-small cell lung cancer (NSCLC): A review of risk factors, diagnosis, and treatment. Medicine (Baltimore), 102(8): e32899. https://doi.org/10.1097/md.0000000000032899
[54] RAMALINGAM SS, VANSTEENKISTE J, PLANCHARD D, CHO BC, GRAY JE, OHE Y, et al. (2020).Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med, 382(1): 41-50. https://doi.org/10.1056/NEJMoa1913662
[55] WANG M, HERBST RS, BOSHOFF C. (2021).Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med, 27(8): 1345-1356. https://doi.org/10.1038/s41591-021-01450-2
[56] KANG R, KROEMER G, TANG D. (2019).The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med, 133: 162-168. https://doi.org/10.1016/j.freeradbiomed.2018.05.074
[57] MAO C, WANG X, LIU Y, WANG M, YAN B, JIANG Y, et al. (2018).A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res, 78(13): 3484-3496. https://doi.org/10.1158/0008-5472.Can-17-3454
[58] ZHANG EB, YIN DD, SUN M, KONG R, LIU XH, YOU LH, et al. (2014).P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis, 5(5): e1243. https://doi.org/10.1038/cddis.2014.201
[59] LU KH, LI W, LIU XH, SUN M, ZHANG ML, WU WQ, et al. (2013).Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer, 13: 461. https://doi.org/10.1186/1471-2407-13-461
[60] XU J, SU C, ZHAO F, TAO J, HU D, SHI A, et al. (2018).Paclitaxel promotes lung cancer cell apoptosis via MEG3-P53 pathway activation. Biochem Biophys Res Commun, 504(1): 123-128. https://doi.org/10.1016/j.bbrc.2018.08.142
[61] HAYANO M, YANG WS, CORN CK, PAGANO NC, STOCKWELL BR. (2016).Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ, 23(2): 270-278. https://doi.org/10.1038/cdd.2015.93
[62] WU H, LIU A. (2021).Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J Int Med Res, 49(3): 300060521996183. https://doi.org/10.1177/0300060521996183
[63] FU M, GU J, JIANG P, QIAN H, XU W, ZHANG X. (2019).Exosomes in gastric cancer: roles, mechanisms, and applications. Mol Cancer, 18(1): 41. https://doi.org/10.1186/s12943-019-1001-7
[64] ZHANG H, WANG M, HE Y, DENG T, LIU R, WANG W, et al. (2021).Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells. Cell Death Dis, 12(12): 1116. https://doi.org/10.1038/s41419-021-04406-z
[65] PéREZ-HERAS AM, MAYNERIS-PERXACHS J, COFáN M, SERRA-MIR M, CASTELLOTE AI, LóPEZ-SABATER C, et al. (2018).Long-chain n-3 PUFA supplied by the usual diet decrease plasma stearoyl-CoA desaturase index in non-hypertriglyceridemic older adults at high vascular risk. Clin Nutr, 37(1): 157-162. https://doi.org/10.1016/j.clnu.2016.11.009
[66] NIU R, ZHAO F, DONG Z, LI Z, LI S. (2022).A stratification system of ferroptosis and iron-metabolism related LncRNAs guides the prediction of the survival of patients with esophageal squamous cell carcinoma. Front Oncol, 12: 1010074. https://doi.org/10.3389/fonc.2022.1010074
[67] CHEN C, ZHAO J, LIU JN, SUN C. (2021).Mechanism and role of the neuropeptide LGI1 receptor ADAM23 in regulating biomarkers of ferroptosis and progression of esophageal cancer. Dis Markers, 2021: 9227897. https://doi.org/10.1155/2021/9227897
[68] YANG H, HU Y, WENG M, LIU X, WAN P, HU Y, et al. (2022).Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. J Adv Res, 37: 91-106. https://doi.org/10.1016/j.jare.2021.10.001
[69] YAO X, YANG P, JIN Z, JIANG Q, GUO R, XIE R, et al. (2019).Multifunctional nanoplatform for photoacoustic imaging-guided combined therapy enhanced by CO induced ferroptosis. Biomaterials, 197: 268-283. https://doi.org/10.1016/j.biomaterials.2019.01.026
[70] HUANG G, XIANG Z, WU H, HE Q, DOU R, LIN Z, et al. (2022).The lncRNA BDNF-AS/WDR5/FBXW7 axis mediates ferroptosis in gastric cancer peritoneal metastasis by regulating VDAC3 ubiquitination. Int J Biol Sci, 18(4): 1415-1433. https://doi.org/10.7150/ijbs.69454
[71] MALDONADO EN, SHELDON KL, DEHART DN, PATNAIK J, MANEVICH Y, TOWNSEND DM, et al. (2013).Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem, 288(17): 11920-11929. https://doi.org/10.1074/jbc.M112.433847
[72] PANG JY, LI PL, AFERIN B, YAN Y, WANG JM. (2021). New strategies for cancer treatment based on ferroptosis. Modern Oncology, 29(06): 1058-1061.
[73] BRAY F, LAVERSANNE M, SUNG H, FERLAY J, SIEGEL RL, SOERJOMATARAM I, et al. (2024).Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 74(3): 229-263. https://doi.org/10.3322/caac.21834
[74] CASADEI C, DIZMAN N, SCHEPISI G, CURSANO MC, BASSO U, SANTINI D, et al. (2019).Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol, 11: 1758835919890285. https://doi.org/10.1177/1758835919890285
[75] HERNANDO POLO S, MORENO MUñOZ D, ROSERO RODRíGUEZ AC, SILVA RUIZ J, ROSERO RODRíGUEZ DI, COUñAGO F. (2021).Changing the History of Prostate Cancer with New Targeted Therapies. Biomedicines, 9(4):392. https://doi.org/10.3390/biomedicines9040392
[76] POSADAS EM, LIMVORASAK S, FIGLIN RA. (2017).Targeted therapies for renal cell carcinoma. Nat Rev Nephrol, 13(8): 496-511. https://doi.org/10.1038/nrneph.2017.82
[77] ZHANG Y, GUO S, WANG S, LI X, HOU D, LI H, et al. (2021).LncRNA OIP5-AS1 inhibits ferroptosis in prostate cancer with long-term cadmium exposure through miR-128-3p/SLC7A11 signaling. Ecotoxicol Environ Saf, 220: 112376. https://doi.org/10.1016/j.ecoenv.2021.112376
[78] LI HJ, LI X, PANG H, PAN JJ, XIE XJ, CHEN W. (2015).Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer. Jpn J Clin Oncol, 45(11): 1055-1063. https://doi.org/10.1093/jjco/hyv132
[79] BUCZKOWSKA J, SZELIGA M. (2023).Two Faces of Glutaminase GLS2 in Carcinogenesis. Cancers (Basel), 15(23). https://doi.org/10.3390/cancers15235566
[80] JIAO K, CHENG J, WANG Q, HAO M. (2024).LncRNA UCA1 enhances NRF2 expression through the m(6)A pathway to mitigate oxidative stress and ferroptosis in aging cardiomyocytes. J Bioenerg Biomembr, 56(6): 607-617. https://doi.org/10.1007/s10863-024-10045-8
[81] LUO W, WANG J, XU W, MA C, WAN F, HUANG Y, et al. (2021).LncRNA RP11-89 facilitates tumorigenesis and ferroptosis resistance through PROM2-activated iron export by sponging miR-129-5p in bladder cancer. Cell Death Dis, 12(11): 1043. https://doi.org/10.1038/s41419-021-04296-1
[82] BROWN CW, AMANTE JJ, CHHOY P, ELAIMY AL, LIU H, ZHU LJ, et al. (2019).Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export. Dev Cell, 51(5): 575-586.e574. https://doi.org/10.1016/j.devcel.2019.10.007
[83] CORADDUZZA D, CONGIARGIU A, CHEN Z, ZINELLU A, CARRU C, MEDICI S. (2023).Ferroptosis and Senescence: A Systematic Review. Int J Mol Sci, 24(4). https://doi.org/10.3390/ijms24043658
[84] MAZHAR M, DIN AU, ALI H, YANG G, REN W, WANG L, et al. (2021).Implication of ferroptosis in aging. Cell Death Discov, 7(1): 149. https://doi.org/10.1038/s41420-021-00553-6
[85] GONG D, CHEN M, WANG Y, SHI J, HOU Y. (2022).Role of ferroptosis on tumor progression and immunotherapy. Cell Death Discov, 8(1): 427. https://doi.org/10.1038/s41420-022-01218-8
[86] LU Y, XIE X, LUO L. (2024).Ferroptosis crosstalk in anti-tumor immunotherapy: molecular mechanisms, tumor microenvironment, application prospects. Apoptosis, 29(11-12): 1914-1943. https://doi.org/10.1007/s10495-024-01997-8
[87] LI Q, CHEN K, ZHANG T, JIANG D, CHEN L, JIANG J, et al. (2023).Understanding sorafenib-induced ferroptosis and resistance mechanisms: Implications for cancer therapy. Eur J Pharmacol, 955: 175913. https://doi.org/10.1016/j.ejphar.2023.175913
[88] PEARSON H, MARSHALL LV, CARCELLER F. (2020).Sorafenib in pediatric hepatocellular carcinoma from a clinician perspective. Pediatr Hematol Oncol, 37(5): 412-423. https://doi.org/10.1080/08880018.2020.1740844
[89] LADD AD, DUARTE S, SAHIN I, ZARRINPAR A. (2024).Mechanisms of drug resistance in HCC. Hepatology, 79(4): 926-940. https://doi.org/10.1097/hep.0000000000000237
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Life Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2025-01-15
Accepted 2025-03-21
Published 2025-03-30


