Abstract
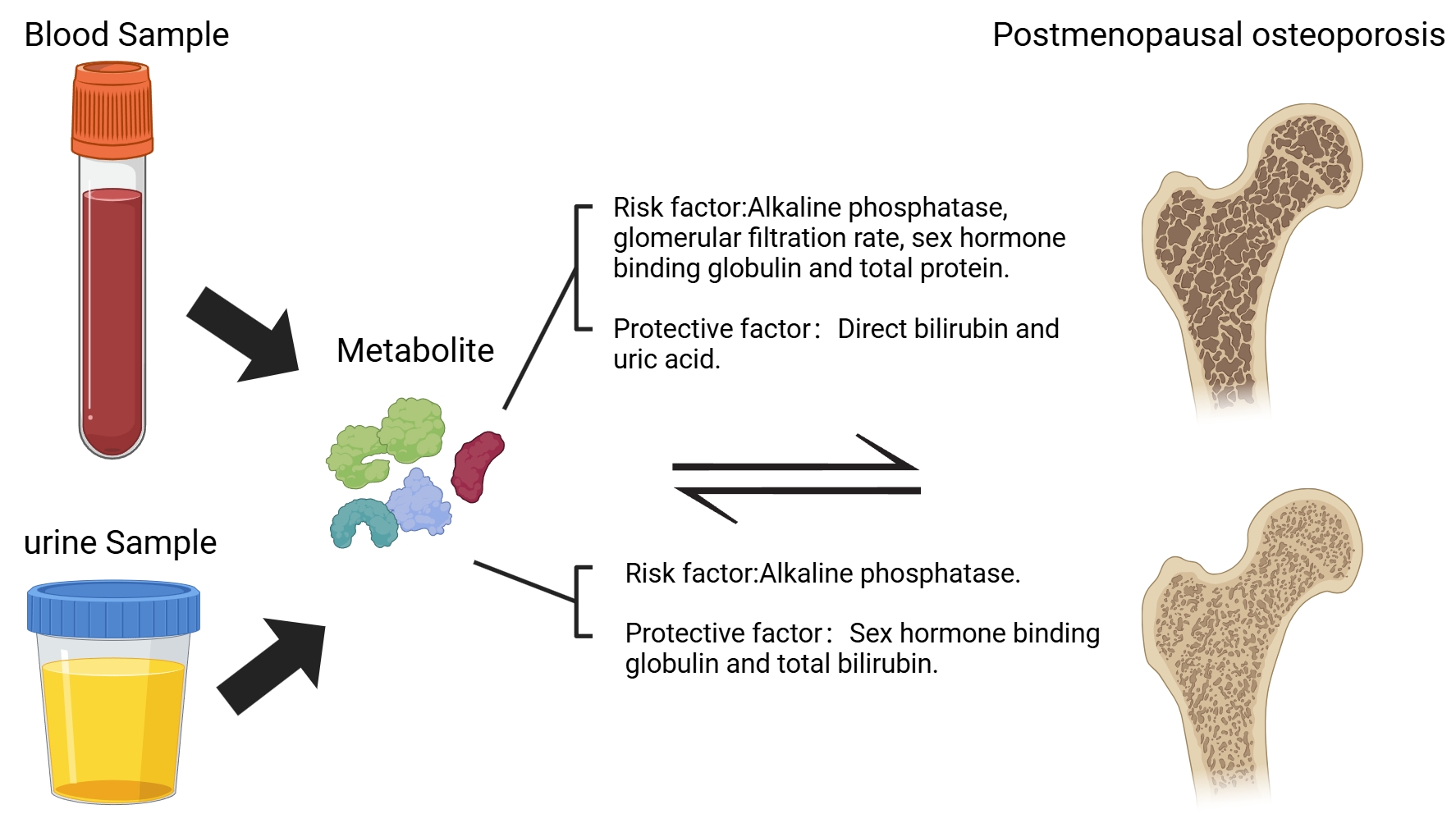
Objective: This study intends to investigate the causal association between 35 blood and urine biomarkers and postmenopausal osteoporosis (PMOP) through two-way Mendelian randomization analysis.
Methods: This study adopted a two-way Mendelian randomization analysis, with data sourced from the UK Biobank and the Finnish Biobank Study. Among them, the R12 dataset of the Finnish Biobank Study was used as the test set, and the R11 dataset as the validation set. The study regarded 35 biomarkers as exposure factors and PMOP (a condition characterized by decreased bone density after menopause) as the outcome variable. It was analyzed through methods such as the inverse variance weighting method, the weighted median method, and MR-Egger regression, and combined with the MR-PRESSO test to exclude the influence of pleiotropy.
Results: In the positive direction analysis, alkaline phosphatase, glomerular filtration rate, sex hormone-binding globulin, and total protein showed statistical significance in both the test set and the validation set, and they were all risk factors for PMOP. Direct bilirubin and uric acid demonstrated statistical significance in both the test and validation sets, and they served as protective factors against PMOP. In the negative direction analysis, alkaline phosphatase showed statistical significance in both the test set and the validation set, being a positive result for PMOP; sex hormone-binding globulin and total bilirubin showed statistical significance in both the test set and the validation set, being negative results for PMOP.
Conclusion: Employing bidirectional Mendelian randomization methodology, this investigation elucidated the causal relationships between multiple hematological and urinary biomarkers and PMOP. The results provide promising biomarker candidates for future diagnostic and therapeutic strategies targeting PMOP, while simultaneously establishing a robust framework for subsequent exploration of its underlying pathophysiological mechanisms.
Keywords: Postmenopausal osteoporosis; Blood and urine biomarkers; Mendelian randomization; Causal association; Metabolite
Introduction
Postmenopausal osteoporosis (PMOP) represents a prevalent metabolic bone disorder predominantly affecting women following menopause, marked by a reduction in bone mineral density and deterioration of bone microarchitecture. This condition substantially elevates fracture susceptibility, profoundly compromising patients' quality of life and longevity [1] . As global demographic trends shift toward an aging population, the prevalence of PMOP continues to escalate annually, emerging as a critical public health concern worldwide [2] .
The pathogenesis of PMOP is complex and involves multiple factors, including estrogen deficiency, imbalance in bone metabolism, genetic factors, and lifestyle, etc. [1-3] . In recent years, biomarkers in blood and urine have demonstrated potential application value in the diagnosis, risk assessment, and treatment monitoring of osteoporosis [4] . For example, biomarkers such as alkaline phosphatase and sex hormone-binding globulin (SHBG) have been confirmed to be closely related to bone metabolism [5,6] . However, the current research on the causal relationship between these biomarkers and PMOP is still insufficient. Most studies are only based on cross-sectional or observational designs, making it difficult to clarify the causal direction.
Mendelian Randomization (MR) represents a robust analytical approach that leverages genetic variants as instrumental variables to infer causal associations, thereby minimizing the impact of confounding variables and bidirectional causation [7] . This methodology has gained significant traction in contemporary research, particularly in elucidating the etiological links between various biomarkers and disease phenotypes. The application of MR has become increasingly prevalent in epidemiological investigations, offering a powerful tool for establishing causal inference in complex biological systems. For example, some studies have revealed the causal associations between various metabolites and chronic diseases such as cardiovascular diseases and diabetes through MR analysis [8-10] . However, there is currently a lack of systematic research on the causal relationship between blood and urine biomarkers and PMOP.
This research employs bidirectional MR to investigate potential causal relationships between 35 circulating and urinary biomarkers and PMOP. Leveraging comprehensive datasets from the UK Biobank and Finnish Biobank studies, this investigation seeks to validate established biomarker-PMOP associations while potentially identifying novel diagnostic indicators. The findings are expected to contribute significantly to advancing our understanding of PMOP pathogenesis, offering valuable insights for diagnostic strategies and therapeutic interventions in this prevalent condition.
Materials and Methods
Study Design
This investigation employed a two-stage analytical approach to examine potential causal relationships between 35 hematological and urinary biomarkers (n=363,228) obtained from the UK Biobank (UKB) [11] and PMOP data derived from the Finnish Biobank Study (FinnGen) [12] . The R12 dataset from FinnGen served as the primary test cohort, while the R11 dataset functioned as the validation cohort. Initial screening for significant associations was conducted through MR analysis using inverse variance weighting (IVW), with biomarkers as exposure variables and PMOP as the outcome measure (significance threshold: P < 0.05). To address potential pleiotropic effects, MR-Egger regression was implemented, retaining associations with P > 0.05. Result consistency was subsequently verified through complementary analyses using the weighted median approach and Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) testing (P > 0.05) [13] . Associations satisfying these rigorous criteria were considered to provide robust evidence of causality [14] .
Instrumental Variables
This investigation employed a rigorous selection process for genome-wide significant single nucleotide polymorphisms (SNPs) associated with the exposure, applying a stringent significance threshold (P < 5 × 10⁻⁸). To minimize potential confounding effects, SNPs exhibiting linkage disequilibrium (LD) were systematically excluded based on established criteria (R² < 0.001, with a clustering distance of 10,000 kb), while maintaining consistency in the effect allele direction. The instrumental variables were required to meet three fundamental criteria: demonstrating robust association with the exposure, independence from confounding variables, and exerting influence on the outcome exclusively through the exposure pathway [7,15] . Instrumental variable strength was quantitatively assessed using the F statistic (F > 10) to eliminate weak associations [16] . Additionally, minor allele frequency (MAF) was derived from effect allele frequency (EAF) calculations, with SNPs demonstrating MAF < 0.01 being excluded to mitigate the impact of rare genetic variations. The final analytical framework incorporated comprehensive SNP formatting and LD pruning procedures (LD threshold 0.001, distance 10,000 kb) to ensure optimal analytical precision.
Statistical analysis
The causal associations between biomarkers and PMOP were investigated using three primary approaches: IVW method, weighted median method, and MR-Egger regression [7,17] . Heterogeneity among the genetic instruments was assessed through the Cochrane Q test, with a significance threshold set at P < 0.05, and the appropriate model (fixed-effect or random-effect) was selected based on the results. Horizontal pleiotropy was evaluated using the MR-Egger intercept test (P < 0.05), while potential pleiotropic SNPs were identified through leave-one-out sensitivity analysis. To examine reverse causality, reverse MR analysis was conducted. Additionally, the MR-PRESSO framework was applied to detect and remove outliers associated with horizontal pleiotropy (P < 0.05), complemented by the MR-Egger method to assess global pleiotropic effects. All statistical procedures were performed using the R programming environment (version 4.4.1).
Results
Positive MR results: 35 blood/urine metabolites as causal validation of the association between exposure and PMOP as an outcome
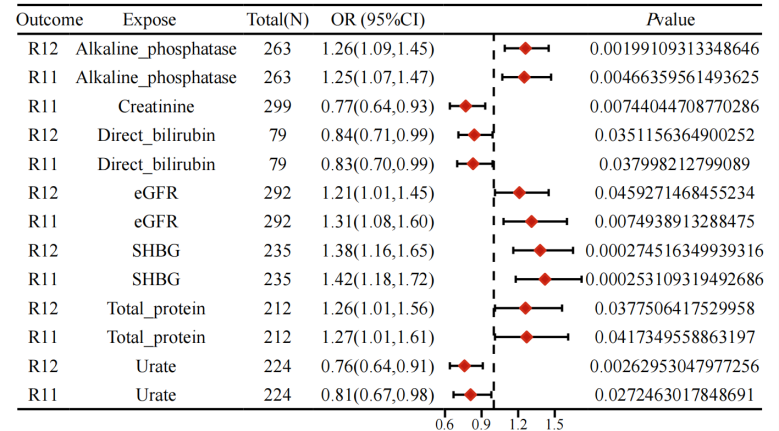
The results of the study showed a batch Mendelian randomisation analysis with a p-value of less than 0.05 for the inverse variance-weighted results and selection of the appropriate mode of interpretation based on heterogeneity. In the test cohort, six significant positive associations were identified: alkaline phosphatase (odds ratio [OR] = 1.26, 95% confidence interval [CI] =1.09-1.45, P=0.002), glomerular filtration rate (OR = 1.21, 95%CI = 1.01-1.45, P = 0.046), sex hormone binding globulin (OR = 1.38, 95%CI = 1.16-1.65, P < 0.001), total protein (OR=1.26, 95%CI = 1.10-1.56, P = 0.038), direct bilirubin (OR = 0.84, 95%CI = 0.71-0.99, P = 0.035), and uric acid (OR = 0.76, 95%CI = 0.64-0.91, P = 0.003). In the validation cohort, seven significant positive associations were observed: alkaline phosphatase (OR = 1.25, 95% CI = 1.07-1.47, P = 0.005), glomerular filtration rate (OR=1.31, 95%CI = 1.08-1.60, P = 0.007), sex hormone binding globulin (OR = 1.42, 95% CI = 1.18-1.72, P < 0.001), total protein (OR = 1.27, 95%CI = 1.01-1.61, P = 0.042), creatinine (OR = 0.77, 95%CI = 0.64-0.93, P = 0.007), direct bilirubin (OR = 0.83, 95%CI = 0.70-0.99, P = 0.027), and uric acid (OR = 0.81, 95%CI = 0.67-0.92, P=0.003). In particular, alkaline phosphatase, glomerular filtration rate, SHBG, and total protein were statistically significant in both the test and validation sets and were risk factors for promoting the development of PMOP, while direct bilirubin and uric acid were statistically significant in both the test and validation sets and were protective factors for inhibiting the development of PMOP (Figure 1). In the MR Egger's method test, all results did not show pleiotropy (P>0.05), whereas MR-Presso showed statistically significant P-values (P < 0.05) before and after correction for SNP at the level of pleiotropy. Detailed data are shown in the Supplementary Material.
Figure1 Forest plot of forward MR results
Inverse MR results: Causal validation between PMOP as exposure and 35 blood/urine metabolites as outcome
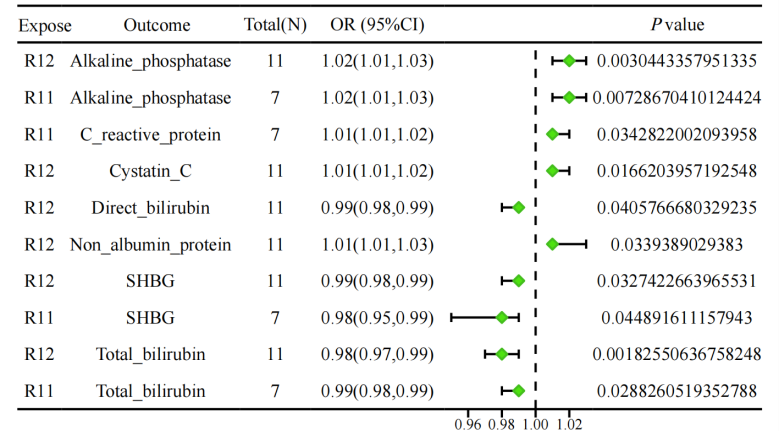
The results were subjected to batch MR analysis, employing a significance threshold of P < 0.05 for inverse variance weighted estimates, with the interpretation method adjusted according to heterogeneity levels. In the initial testing cohort, six significant associations were identified: alkaline phosphatase (OR=1.02, 95% CI=1.01-1.03, P=0.003), cystatin C (OR=1.20, 95% CI=1.01-1.02, P=0.017), direct bilirubin (OR=0.99, 95% CI=0.98-0.99, P=0.041), non-albumin proteins (OR=1.01, 95% CI=1.01-1.03, P=0.038), SHBG (OR=0.99, 95% CI=0.98-0.99, P=0.033), and total bilirubin (OR=0.98, 95% CI=0.97-0.99, P=0.002). In the validation cohort, four significant associations were confirmed: alkaline phosphatase (OR=1.02, 95% CI=1.01-1.03, P=0.0057), C-reactive protein (OR=1.01, 95% CI=1.01-1.02, P=0.034), and SHBG (OR=0.98, 95% CI=0.95-0.99, P=0.029). Among them, alkaline phosphatase was statistically significant in both the test and validation sets and was a positive outcome for the occurrence of PMOP, while SHBG and total bilirubin were statistically significant in both the test and validation sets and were a negative outcome for the occurrence of PMOP (Figure 2).None of the results showed multiplicity in the test of the MR Egger's method (P > 0.05), and the MR-Presso showed statistically significant P-values before and after correction for SNP at the level of pleiotropy (P < 0.05). Detailed data are shown in the Supplementary Material.
Figure2 Forest plot of inverse MR results
Discussion
This research represents the inaugural investigation into the bidirectional causal relationships between 35 distinct blood and urine biomarkers and PMOP (PMOP) through MR analysis. The study identified significant causal associations between specific biomarkers and PMOP development, offering novel insights into the disease's pathogenesis and establishing a foundation for identifying potential biomarker targets.
Through comprehensive analyses, alkaline phosphatase, glomerular filtration rate, SHBG, and total protein were identified as significant risk factors for PMOP. Conversely, direct bilirubin and uric acid demonstrated protective effects against PMOP development. These findings align with existing literature on biomarker involvement in bone metabolism regulation. Specifically, alkaline phosphatase serves as a crucial marker of bone turnover, with elevated concentrations typically indicating enhanced bone resorption and formation activities, potentially predisposing individuals to PMOP . Furthermore, alterations in SHBG concentrations may influence the biological activity of sex hormones, which are pivotal in the pathogenesis of PMOP [5,6] .
Emerging evidence from clinical investigations has identified several protective biomarkers, including direct bilirubin and uric acid, which potentially influence skeletal homeostasis through their roles in oxidative stress modulation and inflammatory pathway regulation. Among these biomarkers, bilirubin, a potent endogenous antioxidant metabolite, exerts its protective effects by neutralizing reactive oxygen species and reducing oxidative damage, a critical pathogenic factor implicated in the progression of osteoporotic conditions [18,19] . The molecular mechanisms underlying bilirubin's protective effects involve its capacity to scavenge free radicals and mitigate oxidative stress-mediated bone resorption, thereby contributing to the maintenance of bone mineral density and structural integrity. In addition, uric acid, as a purine metabolite, also possesses antioxidant capacity, and its protective effect on bone may be related to the inhibition of inflammatory response and modulation of osteoblast activity [20,21] .
In reverse analyses, alkaline phosphatase, SHBG and total bilirubin showed causal associations with PMOP. This suggests that these biomarkers may not only be risk factors for PMOP, but may also be influenced by osteoporotic status. This bidirectional causality suggests that we need to consider the dynamics of biomarkers and their complex interactions with bone health in a comprehensive manner in clinical practice [22] .
The bidirectional relationship between SHBG and PMOP may reflect a feedback loop: elevated SHBG reduces bioavailable estrogen, exacerbating bone loss, while osteoporosis-induced inflammatory signals (e.g., IL-6) may further suppress SHBG synthesis in the liver. This hypothesis aligns with recent experimental evidence demonstrating IL-6-mediated downregulation of SHBG in hepatocyte models [23] .
Notably, alkaline phosphatase was a risk factor for PMOP in the forward analysis and increased with PMOP in the reverse analysis, suggesting a possible positive feedback mechanism between alkaline phosphatase and PMOP. SHBG was a risk factor for promoting the development of PMOP in the forward analysis, whereas the opposite was true in the reverse analysis, and its level may be suppressed with the development of PMOP.
Although this study used multiple Mendelian randomisation methods to reduce the effects of pleiotropy and confounding, several limitations remain. Firstly, Mendelian randomisation analyses rely on the strength and validity of genetic instrumental variables, and although we used methods such as the F-statistic and MR-PRESSO to assess this, there may still be pleiotropy that has not been fully excluded [16] . Secondly, only 35 biomarkers were analysed in this study, while other potential metabolites or biomarkers may also be causally associated with PMOP, and future studies need to further extend the analysis. While our findings provide valuable insights into the causal associations between biomarkers and PMOP, the generalizability of results may be limited by the predominantly European ancestry of participants in both UK Biobank and FinnGen datasets. Future studies incorporating diverse populations (e.g., Asian or African cohorts) are warranted to validate these associations across ethnic groups. Furthermore, it is imperative to conduct validation studies across multiple independent cohorts to confirm the robustness and reproducibility of the results, thereby enhancing their generalizability and reliability.
Conclusion
In this study, the causal associations between multiple blood and urine biomarkers and PMOP were revealed by bidirectional Mendelian randomization analysis. These findings not only provide potential biomarker targets for the diagnosis and treatment of PMOP, but also provide new directions for further research on its pathogenesis. Future studies should further explore the potential mechanisms of these biomarkers and validate their causal associations in more populations, with the aim of providing a stronger basis for the prevention and treatment of postmenopausal osteoporosis.
Abbreviations
PMOP, Postmenopausal osteoporosis; MR, Mendelian Randomization; UKB, UK Biobank; FinnGen, Finnish Biobank Study; IVW, Inverse Variance Weighting; MR-PRESSO, Mendelian Randomization Pleiotropy RESidual Sum and Outlier; SNP, Single Nucleotide Polymorphism; MAF, Minor Allele Frequency; EAF, Effect Allele Frequency; LD, Linkage Disequilibrium; OR, Odds Ratio; CI, Confidence Interval; SHBG, Sex Hormone-binding Globulin.
Supplementary Material
Supplementary methods, results, spectra, figures.
Acknowledgements
Not applicable.
Author contributions
Yimin Liu designed the whole project and was responsible for the software. Runtong Liu curated the data and provided resources. Yuhan Zhao conducted the formal analysis and supervised the project. Yongheng Wang carried out the investigation. Xiaoli Hou was in charge of the methodology. Lei Xing administered the project. Fuyuan Cao was responsible for the validation. Xinhao Fan and Binbin An handled the visualization. All authors contributed to writing the original draft and the review and editing of the manuscript.
Ethics approval and consent to participate
The research did not involve any human participants or animals, and therefore did not require approval from an ethics committee. All data used in this study were obtained from publicly available sources and were analyzed in accordance with ethical guidelines and regulations.
Funding information
Not applicable.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
The data supporting this study are included in the manuscript or its supplementary materials. Publicly accessible datasets were utilized for this analysis and are available at the following repositories: (https://www.finngen.fi/en) and (https://www.ukbiobank.ac.uk/).
References
[1] Arceo-Mendoza RM, & Camacho PM. (2021). Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol Metab Clin North Am, 50(2), 167-178. https://doi.org/10.1016/j.ecl.2021.03.009
[2] Walker MD, & Shane E. (2023). Postmenopausal Osteoporosis. N Engl J Med, 389(21), 1979-1991. https://doi.org/10.1056/NEJMcp2307353
[3] Zhang L, Zheng YL, Wang R, Wang XQ, & Zhang H. (2022). Exercise for osteoporosis: A literature review of pathology and mechanism. Front Immunol, 13, 1005665. https://doi.org/10.3389/fimmu.2022.1005665
[4] Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, & Giorgino R. (2021). Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res, 16(1), 351. https://doi.org/10.1186/s13018-021-02497-0
[5] Marriott RJ, Murray K, Adams RJ, Antonio L, Ballantyne CM, Bauer DC, et al. (2023). Factors Associated With Circulating Sex Hormones in Men : Individual Participant Data Meta-analyses. Ann Intern Med, 176(9), 1221-1234. https://doi.org/10.7326/m23-0342
[6] Sadhukhan S, Sethi S, Rajender S, Mithal A, & Chattopadhyay N. (2023). Understanding the characteristics of idiopathic osteoporosis by a systematic review and meta-analysis. Endocrine, 82(3), 513-526. https://doi.org/10.1007/s12020-023-03505-5
[7] Sekula P, Del Greco MF, Pattaro C, & Köttgen A. (2016). Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol, 27(11), 3253-3265. https://doi.org/10.1681/asn.2016010098
[8] Li J, Wang W, Liu F, Qiu L, Ren Y, Li M, et al. (2024). Genetically predicted 1091 blood metabolites and 309 metabolite ratios in relation to risk of type 2 diabetes: a Mendelian randomization study. Front Genet, 15, 1356696. https://doi.org/10.3389/fgene.2024.1356696
[9] Xu M, Zheng J, Hou T, Lin H, Wang T, Wang S, et al. (2022). SGLT2 Inhibition, Choline Metabolites, and Cardiometabolic Diseases: A Mediation Mendelian Randomization Study. Diabetes Care, 45(11), 2718-2728. https://doi.org/10.2337/dc22-0323
[10] Pan T, Bai L, Zhu D, Wei Y, Zhao Q, Feng F, et al. (2024). The causal relationship between genetically predicted blood metabolites and idiopathic pulmonary fibrosis: A bidirectional two-sample Mendelian randomization study. PLoS One, 19(4), e0300423. https://doi.org/10.1371/journal.pone.0300423
[11] Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. (2021). Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet, 53(2), 185-194. https://doi.org/10.1038/s41588-020-00757-z
[12] Fan Z, Zhao J, Chen J, Hu W, Ma J, & Ma X. (2024). Causal associations of osteoporosis with stroke: a bidirectional Mendelian randomization study. Osteoporos Int, 35(12), 2127-2135. https://doi.org/10.1007/s00198-024-07235-w
[13] Chen D, Xu W, Wen Y, Tan X, & Liu J. (2024). Causal relationship analysis between 35 blood/urine metabolites and gastroesophageal reflux disease: A Mendelian randomization combined meta-analysis study. Medicine (Baltimore), 103(32), e39248. https://doi.org/10.1097/md.0000000000039248
[14] An W, Zhao C, Wang Y, Zhang Y, & Qiao Z. (2024). Identifying causal relationships between 35 blood and urine biomarkers and urologic cancers: MR-meta combined with Bayesian colocalization Mendelian randomization analysis. Discov Oncol, 15(1), 617. https://doi.org/10.1007/s12672-024-01493-0
[15] Birney E. (2022). Mendelian Randomization. Cold Spring Harb Perspect Med, 12(4). https://doi.org/10.1101/cshperspect.a041302
[16] Pierce BL, Ahsan H, & Vanderweele TJ. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol, 40(3), 740-752. https://doi.org/10.1093/ije/dyq151
[17] Burgess S, & Thompson SG. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 32(5), 377-389. https://doi.org/10.1007/s10654-017-0255-x
[18] Vitek L, Hinds TD, Jr., Stec DE, & Tiribelli C. (2023). The physiology of bilirubin: health and disease equilibrium. Trends Mol Med, 29(4), 315-328. https://doi.org/10.1016/j.molmed.2023.01.007
[19] Kimball JS, Johnson JP, & Carlson DA. (2021). Oxidative Stress and Osteoporosis. J Bone Joint Surg Am, 103(15), 1451-1461. https://doi.org/10.2106/jbjs.20.00989
[20] Xu R, Lian D, Xie Y, Mu L, Wu Y, Chen Z, et al. (2023). Relationship between serum uric acid levels and osteoporosis. Endocr Connect, 12(11). https://doi.org/10.1530/ec-23-0040
[21] Yang K, Li J, & Tao L. (2022). Purine metabolism in the development of osteoporosis. Biomed Pharmacother, 155, 113784. https://doi.org/10.1016/j.biopha.2022.113784
[22] Feng XJ, Zhou WJ, Zhang J, Zhang YD, Yu XN, & Yu F. (2024). [Research progress of novel bone turnover markers in osteoporosis]. Zhonghua Yu Fang Yi Xue Za Zhi, 58(12), 2045-2055. https://doi.org/10.3760/cma.j.cn112150-20240710-00556
[23] Zhao S, Gu J, Tian Y, Wang R, & Li W. (2024). Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms. Open Life Sci, 19(1), 20220822. https://doi.org/10.1515/biol-2022-0822
 Figures
Figures References
References Peer
Peer Information
InformationFigure1 Forest plot of forward MR results

Figure2 Forest plot of inverse MR results

[1] Arceo-Mendoza RM, & Camacho PM. (2021). Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol Metab Clin North Am, 50(2), 167-178. https://doi.org/10.1016/j.ecl.2021.03.009
[2] Walker MD, & Shane E. (2023). Postmenopausal Osteoporosis. N Engl J Med, 389(21), 1979-1991. https://doi.org/10.1056/NEJMcp2307353
[3] Zhang L, Zheng YL, Wang R, Wang XQ, & Zhang H. (2022). Exercise for osteoporosis: A literature review of pathology and mechanism. Front Immunol, 13, 1005665. https://doi.org/10.3389/fimmu.2022.1005665
[4] Migliorini F, Maffulli N, Spiezia F, Peretti GM, Tingart M, & Giorgino R. (2021). Potential of biomarkers during pharmacological therapy setting for postmenopausal osteoporosis: a systematic review. J Orthop Surg Res, 16(1), 351. https://doi.org/10.1186/s13018-021-02497-0
[5] Marriott RJ, Murray K, Adams RJ, Antonio L, Ballantyne CM, Bauer DC, et al. (2023). Factors Associated With Circulating Sex Hormones in Men : Individual Participant Data Meta-analyses. Ann Intern Med, 176(9), 1221-1234. https://doi.org/10.7326/m23-0342
[6] Sadhukhan S, Sethi S, Rajender S, Mithal A, & Chattopadhyay N. (2023). Understanding the characteristics of idiopathic osteoporosis by a systematic review and meta-analysis. Endocrine, 82(3), 513-526. https://doi.org/10.1007/s12020-023-03505-5
[7] Sekula P, Del Greco MF, Pattaro C, & Köttgen A. (2016). Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J Am Soc Nephrol, 27(11), 3253-3265. https://doi.org/10.1681/asn.2016010098
[8] Li J, Wang W, Liu F, Qiu L, Ren Y, Li M, et al. (2024). Genetically predicted 1091 blood metabolites and 309 metabolite ratios in relation to risk of type 2 diabetes: a Mendelian randomization study. Front Genet, 15, 1356696. https://doi.org/10.3389/fgene.2024.1356696
[9] Xu M, Zheng J, Hou T, Lin H, Wang T, Wang S, et al. (2022). SGLT2 Inhibition, Choline Metabolites, and Cardiometabolic Diseases: A Mediation Mendelian Randomization Study. Diabetes Care, 45(11), 2718-2728. https://doi.org/10.2337/dc22-0323
[10] Pan T, Bai L, Zhu D, Wei Y, Zhao Q, Feng F, et al. (2024). The causal relationship between genetically predicted blood metabolites and idiopathic pulmonary fibrosis: A bidirectional two-sample Mendelian randomization study. PLoS One, 19(4), e0300423. https://doi.org/10.1371/journal.pone.0300423
[11] Sinnott-Armstrong N, Tanigawa Y, Amar D, Mars N, Benner C, Aguirre M, et al. (2021). Genetics of 35 blood and urine biomarkers in the UK Biobank. Nat Genet, 53(2), 185-194. https://doi.org/10.1038/s41588-020-00757-z
[12] Fan Z, Zhao J, Chen J, Hu W, Ma J, & Ma X. (2024). Causal associations of osteoporosis with stroke: a bidirectional Mendelian randomization study. Osteoporos Int, 35(12), 2127-2135. https://doi.org/10.1007/s00198-024-07235-w
[13] Chen D, Xu W, Wen Y, Tan X, & Liu J. (2024). Causal relationship analysis between 35 blood/urine metabolites and gastroesophageal reflux disease: A Mendelian randomization combined meta-analysis study. Medicine (Baltimore), 103(32), e39248. https://doi.org/10.1097/md.0000000000039248
[14] An W, Zhao C, Wang Y, Zhang Y, & Qiao Z. (2024). Identifying causal relationships between 35 blood and urine biomarkers and urologic cancers: MR-meta combined with Bayesian colocalization Mendelian randomization analysis. Discov Oncol, 15(1), 617. https://doi.org/10.1007/s12672-024-01493-0
[15] Birney E. (2022). Mendelian Randomization. Cold Spring Harb Perspect Med, 12(4). https://doi.org/10.1101/cshperspect.a041302
[16] Pierce BL, Ahsan H, & Vanderweele TJ. (2011). Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol, 40(3), 740-752. https://doi.org/10.1093/ije/dyq151
[17] Burgess S, & Thompson SG. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol, 32(5), 377-389. https://doi.org/10.1007/s10654-017-0255-x
[18] Vitek L, Hinds TD, Jr., Stec DE, & Tiribelli C. (2023). The physiology of bilirubin: health and disease equilibrium. Trends Mol Med, 29(4), 315-328. https://doi.org/10.1016/j.molmed.2023.01.007
[19] Kimball JS, Johnson JP, & Carlson DA. (2021). Oxidative Stress and Osteoporosis. J Bone Joint Surg Am, 103(15), 1451-1461. https://doi.org/10.2106/jbjs.20.00989
[20] Xu R, Lian D, Xie Y, Mu L, Wu Y, Chen Z, et al. (2023). Relationship between serum uric acid levels and osteoporosis. Endocr Connect, 12(11). https://doi.org/10.1530/ec-23-0040
[21] Yang K, Li J, & Tao L. (2022). Purine metabolism in the development of osteoporosis. Biomed Pharmacother, 155, 113784. https://doi.org/10.1016/j.biopha.2022.113784
[22] Feng XJ, Zhou WJ, Zhang J, Zhang YD, Yu XN, & Yu F. (2024). [Research progress of novel bone turnover markers in osteoporosis]. Zhonghua Yu Fang Yi Xue Za Zhi, 58(12), 2045-2055. https://doi.org/10.3760/cma.j.cn112150-20240710-00556
[23] Zhao S, Gu J, Tian Y, Wang R, & Li W. (2024). Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms. Open Life Sci, 19(1), 20220822. https://doi.org/10.1515/biol-2022-0822
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Life Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2024-11-15
Accepted 2024-12-25
Published 2024-12-30


