Abstract
Objective: The aim is to analyze the current research status and hotspots of squamous cell carcinoma endothelial cells and to provide a reference for the following fundamental research and clinical treatment.
Methods: The Web of Science Core Collection (WOSCC) database was searched for literature on squamous cell carcinoma endothelial cells from January 1, 2004, to December 31, 2023. The results were analyzed for research trends, authors, countries, research institutions, and keywords using CiteSpace, VOSviewer, and the bibliometrixc data package in R language.
Results: 3217 articles were included in the analysis, and the number of articles published in the past five years is relatively stable. The number of publications from 89 countries and 3,163 institutions has been relatively stable in the past five years, of which 800 are from China, 195 are from the University of Texas System, which is higher than that of other countries and institutions, and there is a big difference in the number of publications between countries and institutions. Prof. Marioni Gino has published 16 relevant papers, and 713 citations have been given to Forkman J, so there is more frequent cooperation between scholars. There are more frequent collaborations among scholars. Eight hundred sixty-eight journals published relevant papers, with Oral Oncology having the highest number of articles and Cancer Research having the highest number of citations. The most cited reference is “Hallmarks of cancer: the next generation,” DOI:10.1016/j.cell.2011.02.013 (intensity 21.75). In the last five years, keywords with high intensity are migration, tumor microenvironment, open-label, epithelial-mesenchymal transition, t cells, esophageal squamous cell carcinoma, and recurrent.
Conclusion: The development of squamous cell carcinoma endothelial cell research is uneven among different countries, institutions, and authors, and the journal Oral Oncology publishes the most relevant papers, with current research hotspots including metastasis, recurrence, tumor microenvironment, epithelial-mesenchymal transition, and open labelling.
Keywords: Squamous cell carcinoma; Endothelial cells; Research progress; Bibliometric analysis; hotspot
Introduction
Squamous cell carcinoma (SCC) is an aggressive malignant tumor commonly found in epithelial tissues of the skin, oral cavity, esophagus, and lungs[1] . As the constitutive cells of blood vessels, endothelial cells play a key role in maintaining tissue homeostasis and play a complex role in tumor angiogenesis, immune escape, and tumor microenvironment regulation[2] .
In recent years, the role of endothelial cells in the development of squamous cell carcinoma has been gradually revealed with the in-depth study of the tumor microenvironment[3] . Endothelial cells are not only involved in tumor angiogenesis but also influence the invasiveness of tumor cells and the infiltration of immune cells by secreting various cytokines and chemokines[4-5] . In addition, aberrant activation of endothelial cells is closely related to tumor metastasis and prognosis, and the exact mechanism of endothelial cells in squamous cell carcinoma is still not fully understood despite the progress of existing studies[6-7] .
Bibliometric analysis is based on the analysis of existing published literature to assess the intrinsic relationships and dissemination patterns in the literature, enabling researchers to quickly understand the hotspots in the field and promote the dissemination and sharing of knowledge[8] . In this study, we used bibliometric analysis to analyze the literature on endothelial cells in squamous cell carcinoma in the last 20 years to provide a reference for future basic research and clinical treatment.
Materials and Methods
Data collection
This data was collected by searching the core database in Web of Science (https://www.webofscience.com/wos/woscc/ basic-search) for literature on squamous cell carcinoma endothelial cells. The search strategy was “TS=(squamous cell carcinoma) AND (Endothelial Cells),” and the literature type was selected as a treatise or review. The period was set from January 1, 2004, to December 31, 2023, and the retrieval was conducted on September 24, 2024. Plain text files, tab-delimited files, and BIBTEX formats were selected for downloading in WOSCC, and the primary information included article title, year of publication, country/region, institution, publication, author, keywords, and cited literature. Two researchers checked and screened the literature after downloading it.
Data analysis
This study used CiteSpace (Version 6.4.R1 Advanced), VOSviewer (1.6.10), and R 4.4.1, GraphPad Prism 10.0 software, to organize and analyze the information in the literature. Where CiteSpace set the period (2004-2023), year slice (1 year), pruning (none), and all other defaults were used, R 4.3.3 chose the bibliometrix packet, and VOSviewer chose the default options.
Results
Annual publication of papers related to squamous cell carcinoma endothelial cells
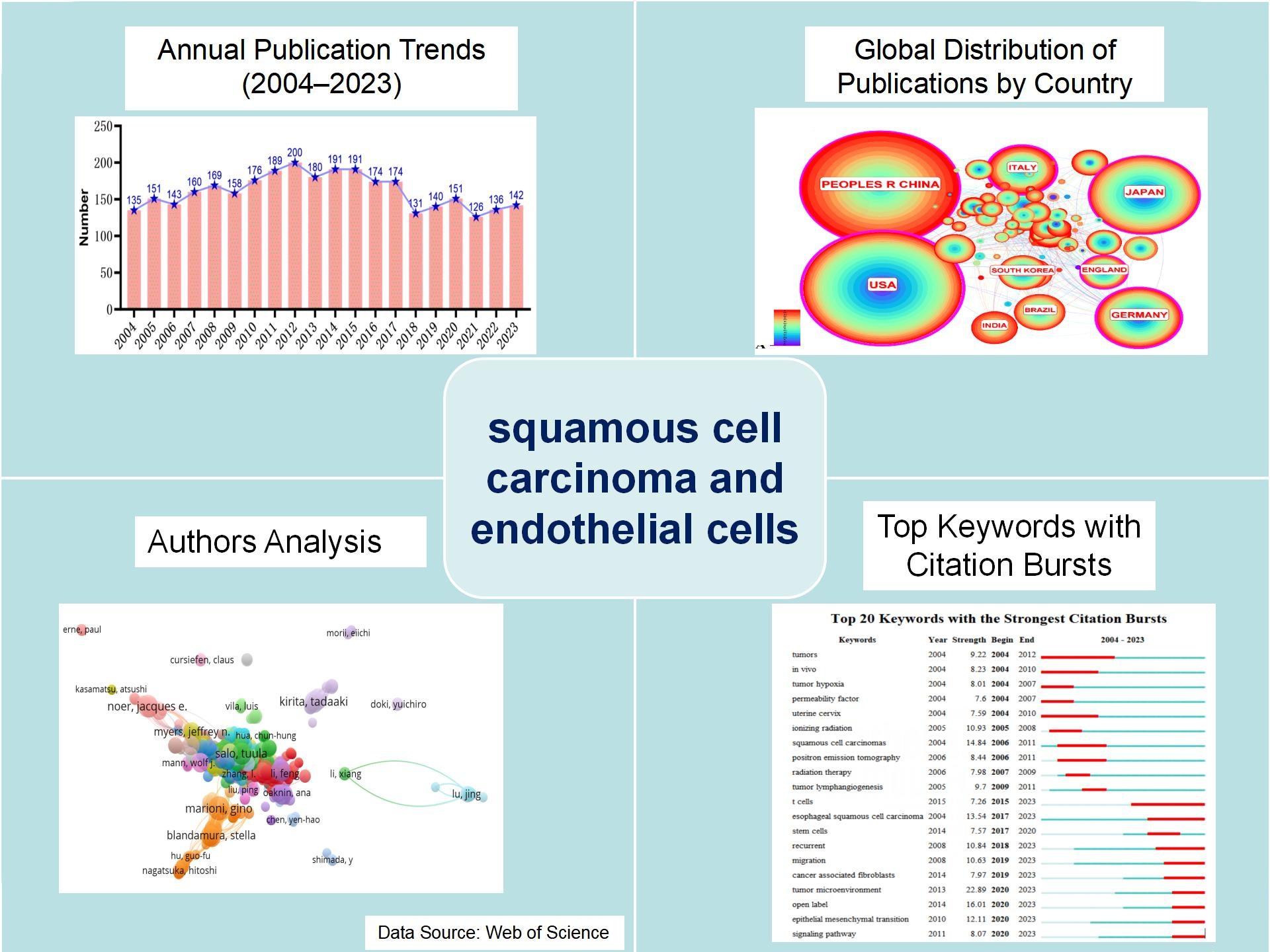
From January 1, 2004, to December 31, 2023, 3217 squamous cell carcinoma endothelial cell treatises and reviews were published. The annual publication volume was generally greater than 120, of which the highest publication volume was 200 in 2012, and the last five years were more stable at 126 to 151 articles(Figure 1).
Figure 1. Annual number of publications related to endothelial cells in squamous cell carcinoma
Distribution of countries and institutions
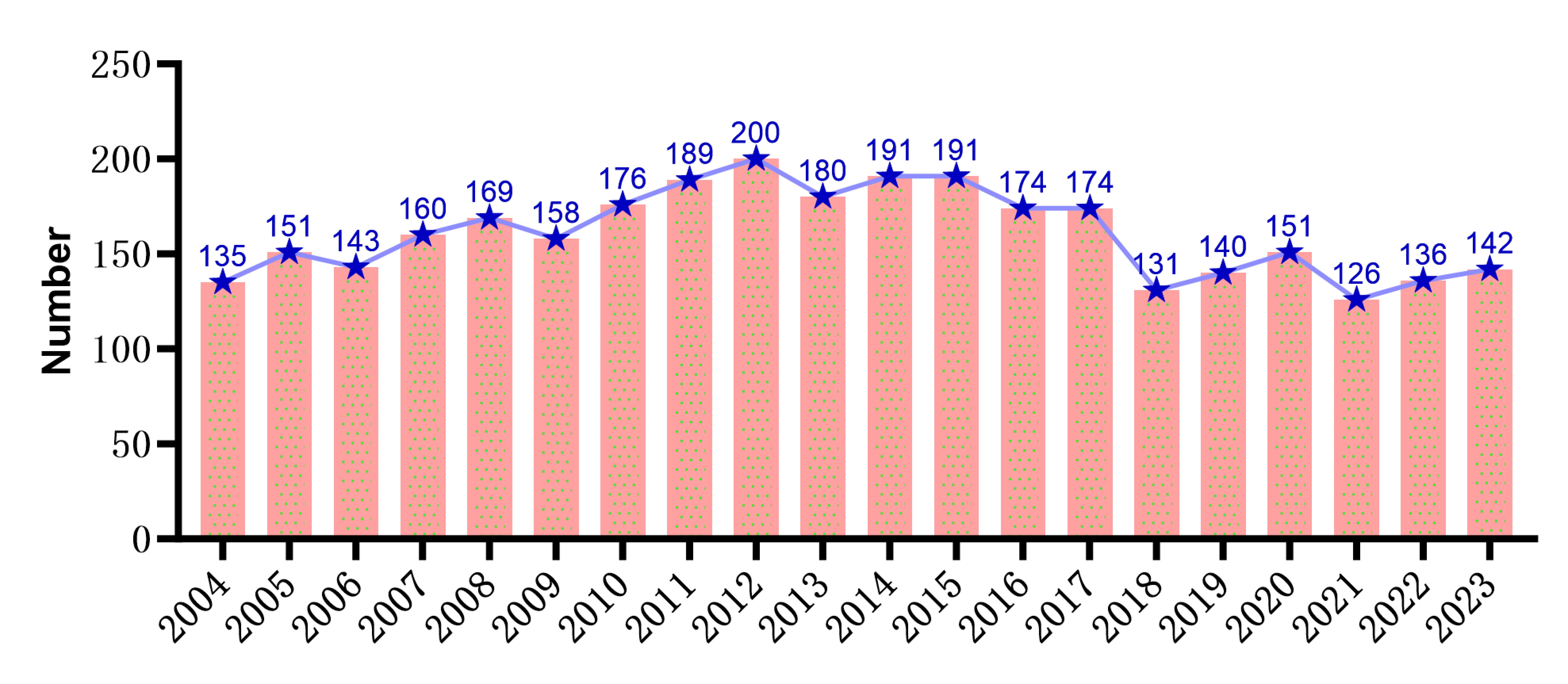
There are 3217 papers from 89 countries and 3163 institutions. The top 3 countries are China (800 papers, centrality 0.13), the United States (795 papers, centrality 0.48), and Japan (408 papers, centrality 0.10), among which China has the highest number of papers, the United States has the highest centrality, and the cooperation among countries is relatively close. The top 3 institutions are the University of Texas System (195 papers, the University of Michigan System (172 papers), and UTMD Anderson Cancer Center (165 papers), and there is a big difference in the number of papers published among institutions. University of Texas System (195 papers), University of Michigan System (172 papers), UTMD Anderson Cancer Center (165 papers), and there is a significant difference in the number of papers published among institutions (Figure 2).
Figure 2. Volume of communications from countries and institutions. (A) Volume of communications from different countries and partnerships. (B) The Volume of communications from different institutions
Authors and co-cited authors
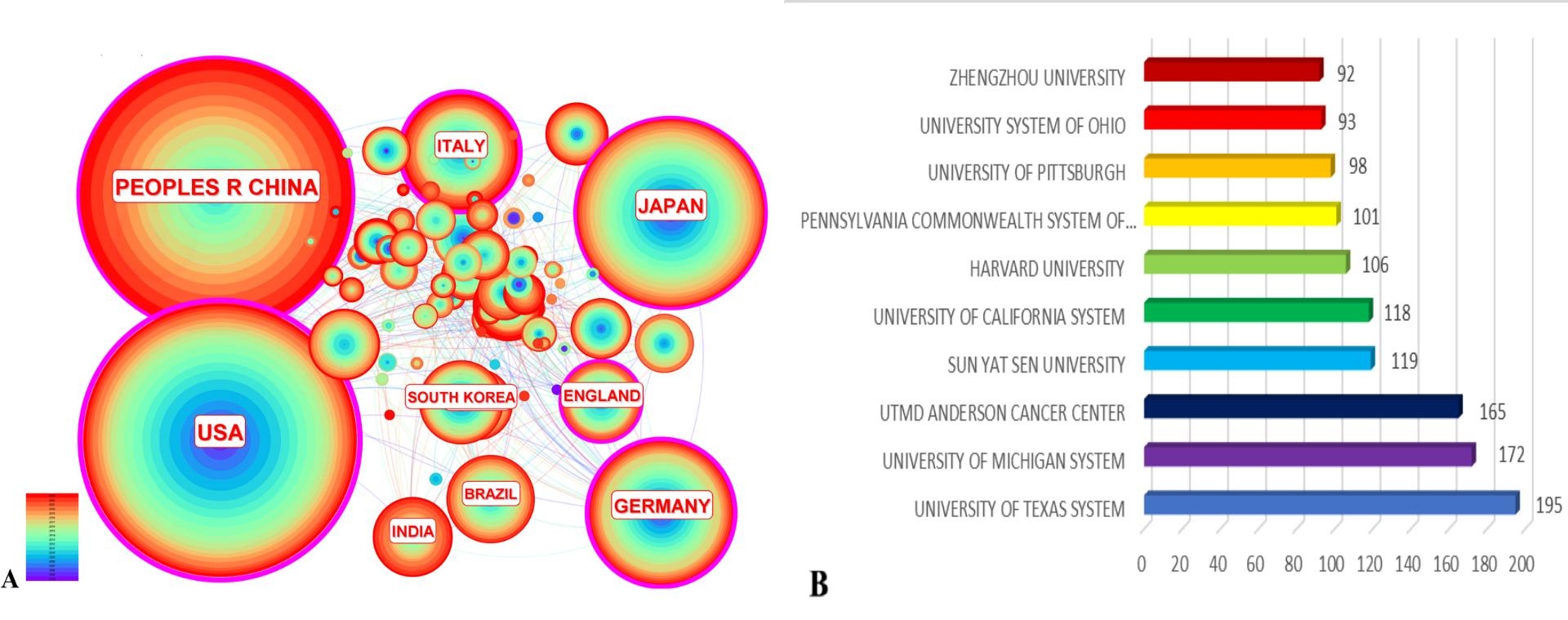
A total of 18,298 authors have published related papers, the top 3 authors are Marioni Gino (16 articles), Neor Jacques e (14 articles), and Kirita Tadaaki (13 articles), and the top 3 authors with the highest number of citations are Forkman J (713), Ferrara N (510), and Hanahan D (356). Three hundred fifty-six times), with more frequent collaborations between individual scholars, Marioni Gino has the highest number of publications, and Forkman J has the highest number of citations (Figure 3).
Figure 3. Authors and cited authors. (A) Clustering plot of authors' publications. (B) Density plot of cited authors
Journals and co-cited journals
A total of 868 journals published papers related to squamous cell carcinoma endothelial cells, with the top 3 journals ranked in the order of 72 articles in Oral Oncology, 64 articles in Anticancer Research, and 59 articles in Oncology Letters, and the top 3 journals ranked in the order of the total number of citations were Cancer Research, Clinical Cancer Research, and Journal of Clinical Oncology, with the top 3 journals ranked in the order of the total number of citations. Clinical Cancer Research, Journal of Clinical Oncology (Figure 4).
Figure 4. Journals and co-cited journals. (A) Clustering plot of author publications. (B) Density plot of cited authors
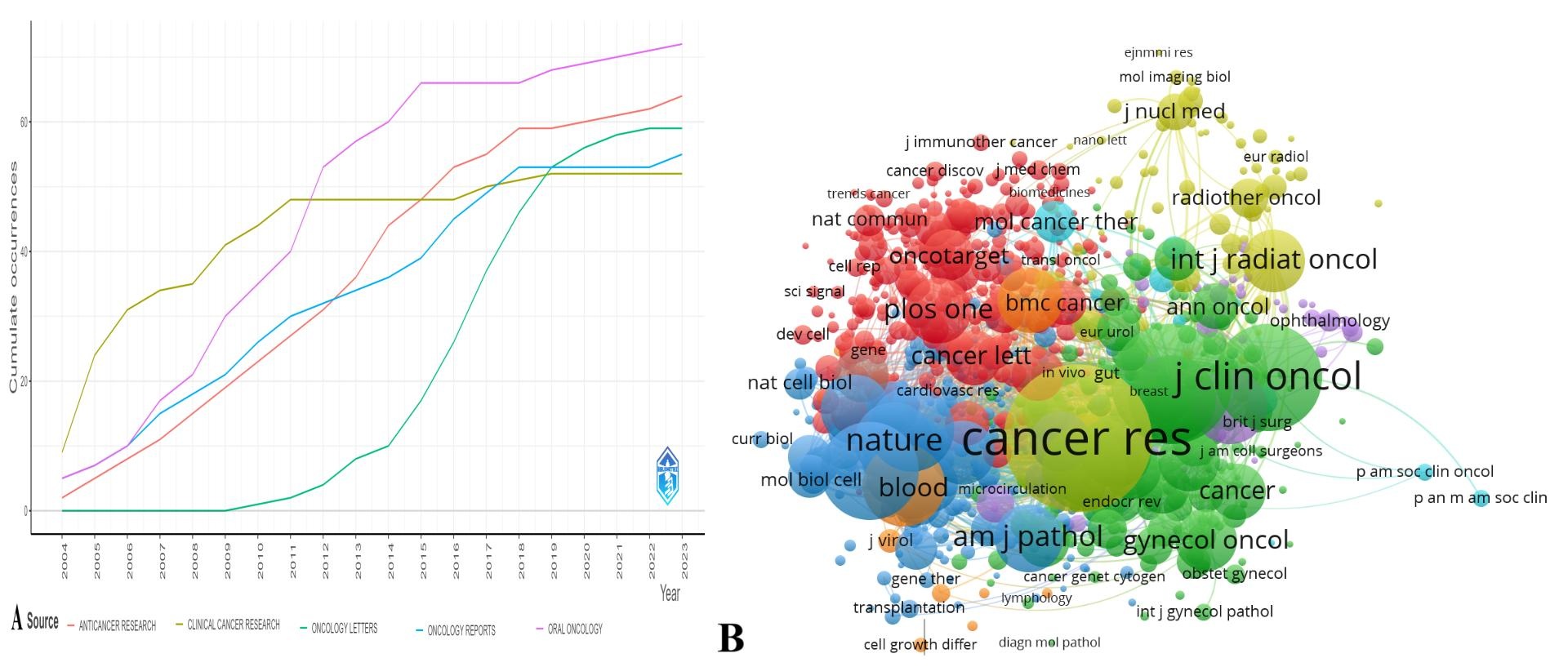
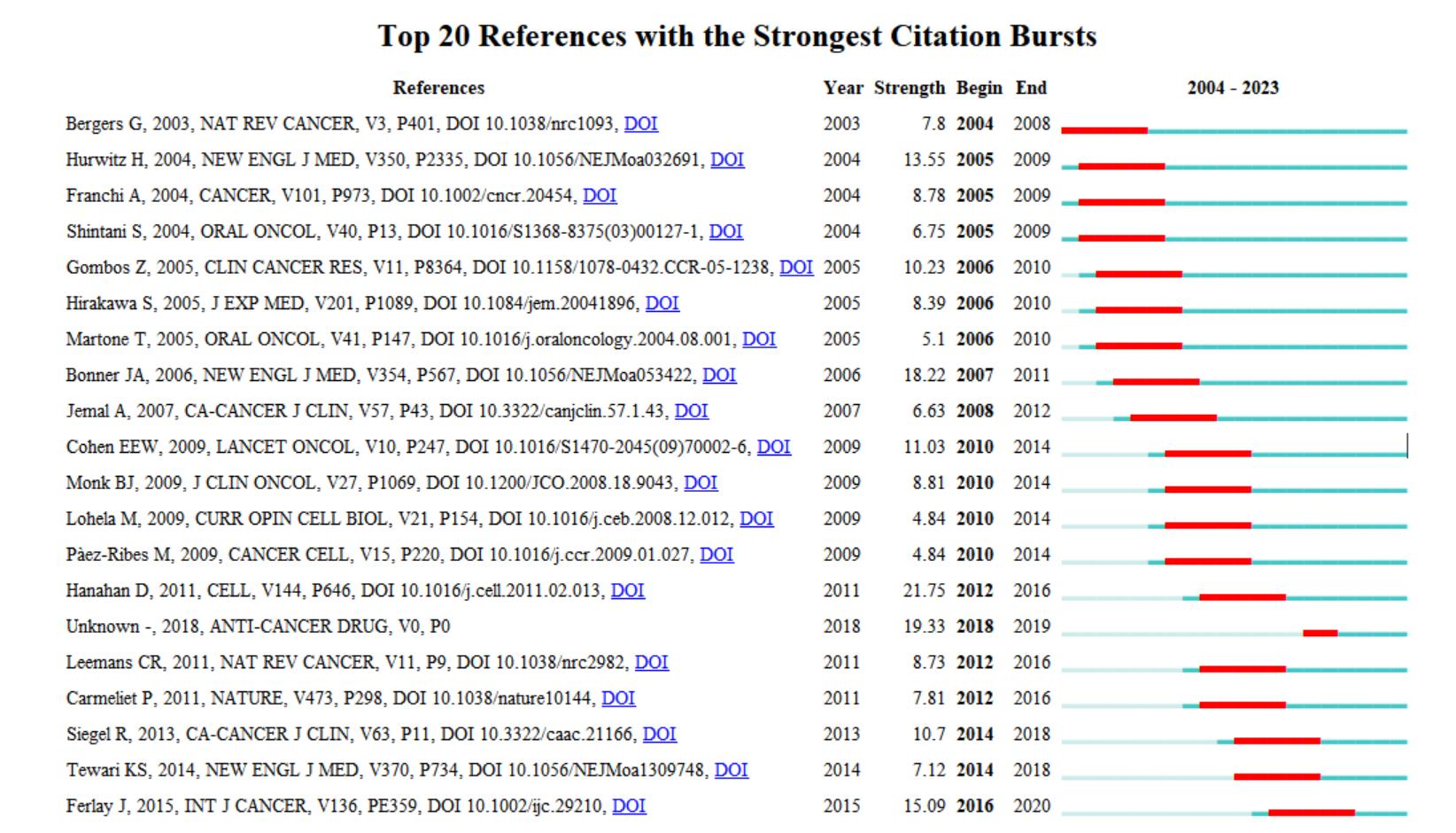
Co-cited references
There are 126,764 cited references, and the top 3 cited references highlighted are, in order, “Hallmarks of cancer: the next generation,” DOI:10.1016/j.cell.2011.02.013 (intensity 21.75), “Thyroid cancer management: from a suspicious nodule to targeted therapy,” DOI:10.1097/CAD.0000000000000617 (intensity 19.33), ”Radiotherapy plus cetuximab for squamous -cell carcinoma of the head and neck,” DOI:10.1056/NEJMoa053422 (intensity 18.22) (Figure 5).
Figure 5. Cited reference highlighting
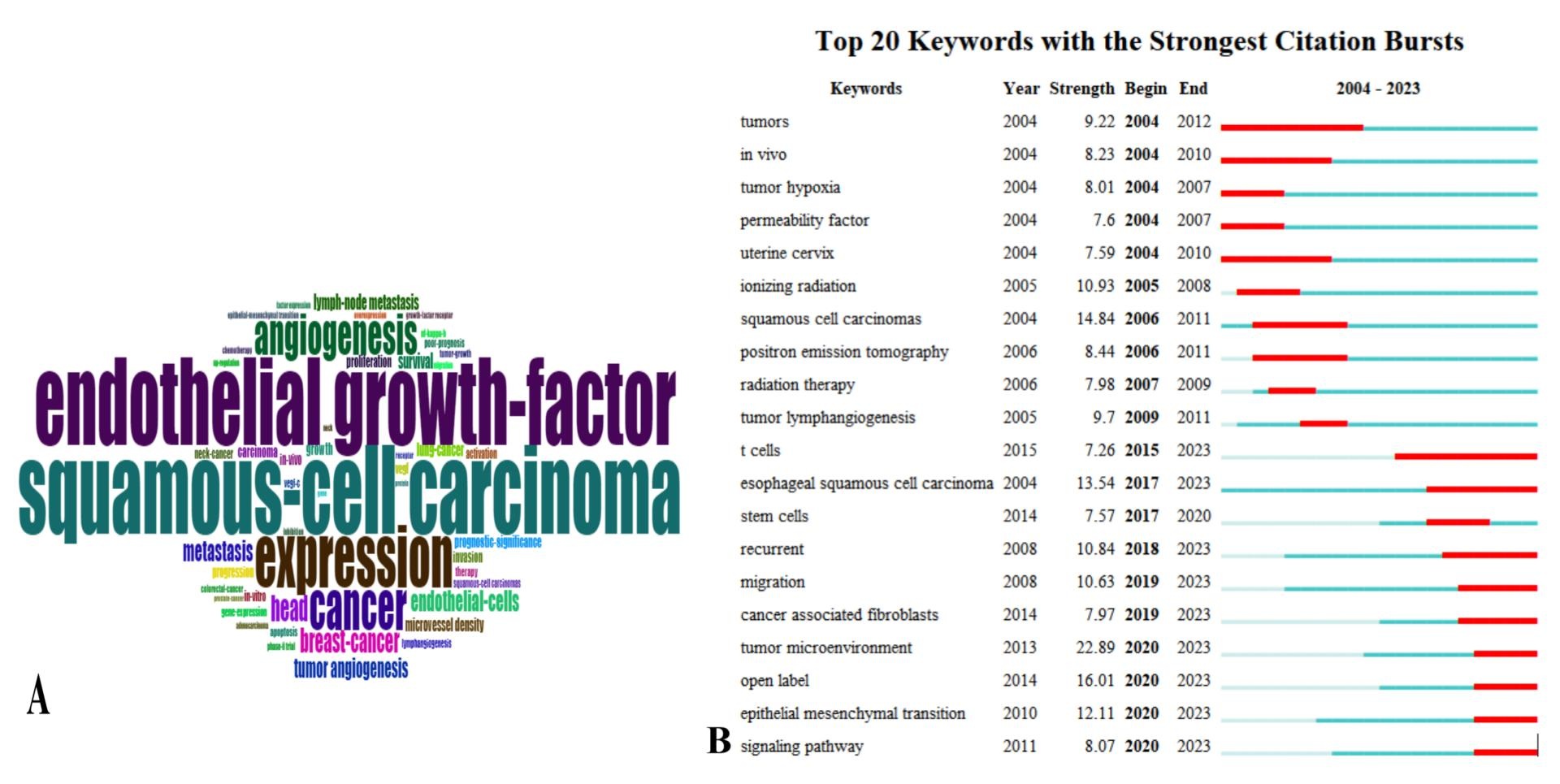
Keyword analysis
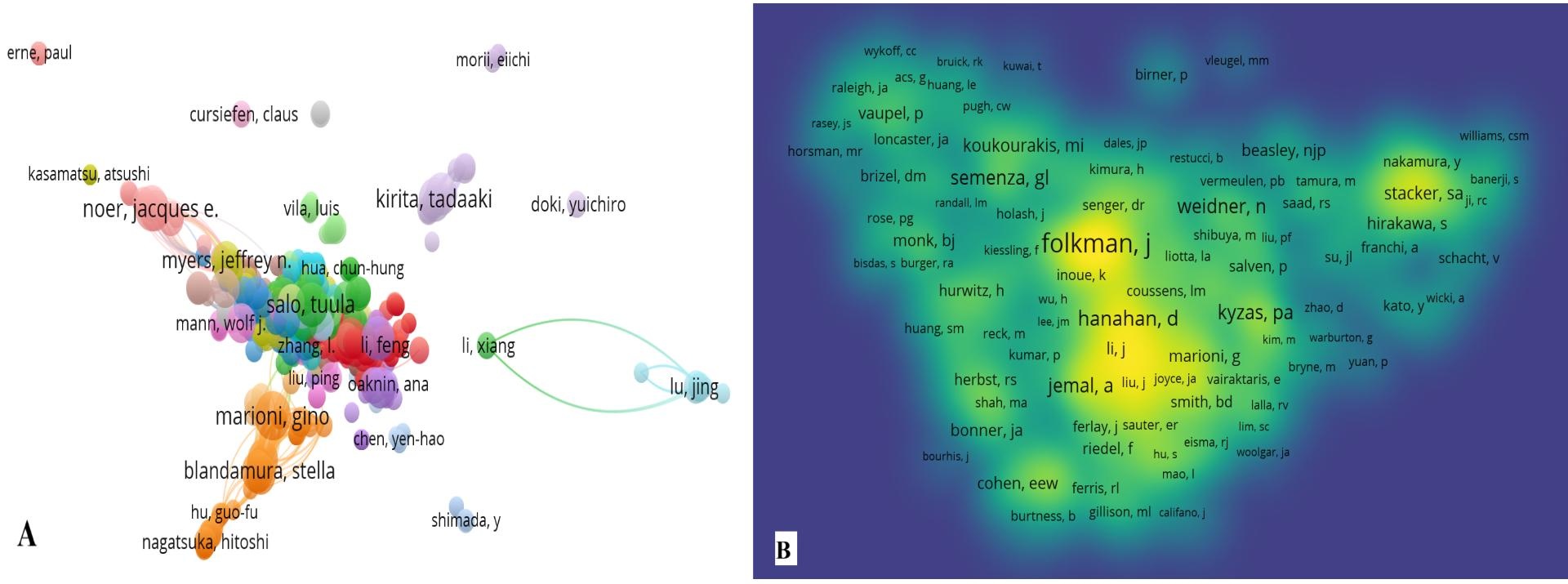
There are 10608 keywords in this analysis, of which the top 3 keywords in order of occurrence are squamous-cell carcinoma (1154 times), endothelial growth factor (1137 times), and expression (810 times); the top 3 keywords in terms of the intensity of keyword emergence display are tumor microenvironment (intensity 22.89), open label (intensity 16.01), squamous cell carcinomas (intensity 14.84), and in the past five years, the keywords with higher intensity are migration, tumor microenvironment, open label, epithelial mesenchymal label, and epithelial mesenchymal label. , epithelial mesenchymal transition, t cells, esophageal squamous cell carcinoma, recurrent (Figure 6).
Figure 6. Keywords as well as keyword emergence. (A) Keyword word cloud map (B) Keyword emergence
Discussion
General Information
A total of 3217 squamous cell carcinoma endothelial cell related literature were included for analysis, with an annual publication volume of more than 120 articles, among which the highest publication volume was 200 articles in 2012, which was reduced in recent years compared with the previous one. The volume of publication volume was relatively stable in the past five years.
Among different countries, China has published 800 articles, and the United States has published 795 articles, far more than other countries. There is active cooperation among countries. However, the difference in the number of articles between countries is more prominent. The University of Texas System has published the most 195 articles among different organizations. In a comprehensive analysis, research teams led by China and the United States have led in squamous cell carcinoma endothelial cell research. However, there are still significant differences between countries and institutions.
In terms of authors, Prof. Marioni Gino has published 16 relevant papers. At the same time, Prof. Forkman J has been cited 713 times, with active collaborations between multiple scholars to promote the common development of the field. Among the journals, Oral Oncology has the most articles, and Cancer Research has the most citations. The literature Hallmarks of Cancer: The Next Generation has the highest number of citations[9] . More journals are publishing papers on squamous cell carcinoma endothelial cells, indicating that this field has received attention from researchers.
Research hotspot
There are 10,608 keywords, and the keywords with higher intensity in the last five years are migration, recurrent, tumor microenvironment, epithelial mesenchymal transition, t cells, open label, and esophageal squamous cell carcinoma.
Endothelial cells play an important role in squamous cell development, and it has been shown that specific circRNA (circFNDC3B) is significantly upregulated in oral squamous cell carcinoma (OSCC) and positively correlates with lymph node metastasis. circFNDC3B can accelerate the migration and invasion of OSCC cells and enhance human umbilical vein endothelial cells and lymphatic vessel endothelial cell tube formation ability[10] . Other studies have shown that endothelin on endothelial cells may play a role in paracrine signalling between cells, leading to the proliferation or migration of squamous cell carcinoma[11-12] . Moreover, in squamous cell carcinoma, circulating endothelial progenitor cells may play a role in regulating anti-tumour immune responses and angiogenesis, thus affecting tumor recurrence and prognosis[13] . Various other cytokines may regulate endothelial cell expression and thus cause tumor recurrence[14] .
The tumor microenvironment plays an important role in squamous cell carcinoma endothelial cells, which can promote tumor angiogenesis by secreting a variety of cytokines and growth factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF)[15-16] . This process is crucial for tumor growth and metastasis as it provides essential nutrients and oxygen to the tumor and helps the tumor cells to evade the immune system surveillance[17] . Epithelial-mesenchymal transition is an important biological process associated with a reduction in intercellular adhesion and an increase in the ability of cells to invade and migrate. In contrast, interactions with endothelial cells may impact the tumor's angiogenic, invasive, and metastatic capacities[18-20] .T cells play an important role in anti-tumor immunity. However, their function may be inhibited by the tumor microenvironment[21] . Modulating T cells' function and endothelial cells' angiogenic capacity may provide new strategies for treating squamous cell carcinoma[22] .
There are many studies on esophageal squamous cell carcinoma and endothelial cells, in which angiogenesis is one of the key factors in tumor growth and metastasis[23] . In esophageal squamous cell carcinoma, VEGF and its signalling pathway are important in promoting angiogenesis. The interactions between esophageal squamous cell carcinoma and endothelial cells are complex and varied, involving various signalling pathways and molecular mechanisms, which provide potential targets for developing new therapies as the pathogenesis continues to be understood[24] . The treatment of squamous cell carcinoma is also a significant concern for researchers, and some of the development and application of new drugs require open-label trials, which can improve the transparency of clinical trials but need to be alert to potential bias[25] .
Limitations
Only papers and reviews published on the Web of Science were included in this study, and not all the data of all the research papers in the world could be included. Meanwhile, this analysis is based on machine learning and natural language processing methods, which may cause operational bias.
Conclusion
In conclusion, the development of squamous cell carcinoma endothelial cell research is uneven among different countries, institutions, and authors, and the journal Oral Oncology publishes the most relevant papers. The current research hotspots include metastasis, recurrence, tumor microenvironment, and open labelling.
Abbreviations
WOSCC: Web of Science Core Collection; SCC: Squamous cell carcinoma; OSCC: Oral squamous cell carcinoma; VEGF: Vascular endothelial growth factor; FGF: Fibroblast growth factor; PDGF: Platelet-derived growth factor.
Acknowledgements
This study was conducted using the web of science database resource. We want to thank all participants.
Author contributions
Research design: Jiang Li, Zhe dong, Wei Ren; Data analysis: Jiang Li, Zhe dong, Sibo Bi, Tianwen Gao; Draft Writing and Revision: Jiang Li, Zhe dong Wei Ren, Junyi Zhou.
Ethics approval and consent to participate
The research did not involve any human participants or animals, and therefore did not require approval from an ethics committee. All data used in this study were obtained from publicly available sources and were analyzed in accordance with ethical guidelines and regulations.
Funding information
Not applicable.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could create a conflict of interest at the time of conducting this study.
Data Availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
References
[1] Liu C, Zhang M, Yan X, Ni Y, Gong Y, Wang C, et al. (2023) Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv. 9(4):eadd8977. https://doi.org/10.1126/sciadv.add8977
[2] Zhang Y, Chen C, Liu Z, Guo H, Lu W, Hu W, et al. (2022) PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 41(1):111. https://doi.org/10.1186/s13046-022-02339-9
[3] Chu T, Wang Z, Pe'er D, Danko CG. (2022). Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat Cancer. 3(4):505-517. https://doi.org/10.1038/s43018-022-00356-3
[4] Sekiguchi S, Yorozu A, Okazaki F, Niinuma T, Takasawa A, Yamamoto E, et al. (2023) ACLP Activates Cancer-Associated Fibroblasts and Inhibits CD8+ T-Cell Infiltration in Oral Squamous Cell Carcinoma. Cancers (Basel). 15(17):4303. https://doi.org/10.3390/cancers15174303
[5] Wei WF, Zhou HL, Chen PY, Huang XL, Huang L, Liang LJ, et al. (2023) Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells. J Exp Clin Cancer Res. 42(1):160. https://doi.org/10.1186/s13046-023-02714-0
[6] Dumitru CS, Ceausu AR, Gaje NP, Suciu CS, Raica M. (2022). Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int J Mol Sci, 23(17):9793. https://doi.org/10.3390/ijms23179793
[7] Dai J, Xi X, Liu Z, Wu W, Zhu S, Zhang X, et al. (2023) Single-cell sequencing of multi-region resolves geospatial architecture and therapeutic target of endothelial cells in esophageal squamous cell carcinoma. Clin Transl Med. 13(11):e1487. https://doi.org/10.1002/ctm2.1487
[8] Long SY, Shang L, Shi H, Zhao S, Cao J, He Y. (2023). The Future Landscape of Endothelial Cells Research in Psoriasis: Bibliometric Analysis and Literature Review. Clin Cosmet Investig Dermatol. 16(1):3107-3120. https://doi.org/10.2147/CCID.S435085
[9] Hanahan D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell. 144(5):646-74. https://doi.org/10.1016/j.cell.2011.02.013
[10] Li X, Wang C, Zhang H, Li Y, Hou D, Liu D, et al. (2023) circFNDC3B Accelerates Vasculature Formation and Metastasis in Oral Squamous Cell Carcinoma. Cancer Res. 83(9):1459-1475. https://doi.org/10.1158/0008-5472.CAN-22-2585
[11] Hakuno SK, Janson SGT, Trietsch MD, de Graaf M, de Jonge-Muller E, Crobach S, et al. (2023) Endoglin and squamous cell carcinomas. Front Med (Lausanne). 10(1):1112573. https://doi.org/10.3389/fmed.2023.1112573
[12] Su NW, Dai SH, Hsu K, Chang KM, Ko CC, Kao CW, et al. (2024) PD-L1-positive circulating endothelial progenitor cells associated with immune response to PD-1 blockade in patients with head and neck squamous cell carcinoma. Cancer Immunol Immunother. 73(1):3. https://doi.org/10.1007/s00262-023-03595-0
[13] Noh JJ, Kim MS, Cho YJ, Jeong SY, Lee YY, Ryu JY, et al. (2020) Anti-Cancer Activity of As4O6 and its Efficacy in a Series of Patient-Derived Xenografts for Human Cervical Cancer. Pharmaceutics. 12(10):987. https://doi.org/10.3390/pharmaceutics12100987
[14] Shimomura H, Sasahira T, Nakashima C, Kurihara-Shimomura M, Kirita T. (2019). Non-SMC Condensin I Complex Subunit H (NCAPH) Is Associated with Lymphangiogenesis and Drug Resistance in Oral Squamous Cell Carcinoma. J Clin Med. 9(1):72. https://doi.org/10.3390/jcm9010072
[15] Li C, Guan R, Li W, Wei D, Cao S, Xu C, et al. (2023) Single-cell RNA sequencing reveals tumor immune microenvironment in human hypopharygeal squamous cell carcinoma and lymphatic metastasis. Front Immunol. 14(1):1168191. https://doi.org/10.3389/fimmu.2023.1168191
[16] Zhang J, Lu T, Lu S, Ma S, Han D, Zhang K, et al. (2022) Single-cell analysis of multiple cancer types reveals differences in endothelial cells between tumors and normal tissues. Comput Struct Biotechnol J. 21(1):665-676. https://doi.org/10.1016/j.csbj.2022.12.049
[17] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[18] Tokozlu B, Yücel ÖÖ, Gültekin SE, Bozdağ LA. (2024). Epithelial mesenchymal transition and cancer stem cell markers in oral epithelial dysplasia and oral squamous cell carcinoma. Pol J Pathol. 75(4):305-314. https://doi.org/10.5114/pjp.2024.145818.
[19] Franz L, Nicolè L, Frigo AC, Ottaviano G, Gaudioso P, Saccardo T, et al. (2021) Epithelial-to-Mesenchymal Transition and Neoangiogenesis in Laryngeal Squamous Cell Carcinoma. Cancers (Basel). 13(13):3339. https://doi.org/10.3390/cancers13133339
[20] Xiao J, Song Y, Gao R, You M, Deng C, Tan G, et al. (2023) Changes of immune microenvironment in head and neck squamous cell carcinoma in 3D-4-culture compared to 2D-4-culture. J Transl Med. 21(1):771. https://doi.org/10.1186/s12967-023-04650-1
[21] He Z, Tian W, Wei Q, Xu J. (2022). Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front Immunol. 13(1):968649. https://doi.org/10.3389/fimmu.2022.968649
[22] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[23] Sun Q, Zhang T, Xiao Q, Mei B, Zhang X. (2022) Procyanidin B2 inhibits angiogenesis and cell growth in oral squamous cell carcinoma cells through the vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2) pathway. Bioengineered. 13(3): 6500-6508. https://doi.org/10.1080/21655979.2022.2033013
[24] Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. (2019) KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet, 394(10212): 1915-1928. https://doi.org/10.1016/S0140-6736(19)32591-7
[25] Ozer M, Sahin I . (2022). Nivolumab in Esophageal Squamous-Cell Carcinoma. N Engl J Med, 386(20):1958-1959. https://doi.org/10.1056/NEJMc2202880
 Figures
Figures References
References Peer
Peer Information
InformationFigure 1. Annual number of publications related to endothelial cells in squamous cell carcinoma

Figure 2. Volume of communications from countries and institutions. (A) Volume of communications from different countries and partnerships. (B) The Volume of communications from different institutions

Figure 3. Authors and cited authors. (A) Clustering plot of authors' publications. (B) Density plot of cited authors

Figure 4. Journals and co-cited journals. (A) Annual publication volume of the top five journals. (B) Density plot of journals.

Figure 5. Cited reference highlighting

Figure 6. Keywords as well as keyword emergence. (A) Keyword word cloud map (B) Keyword emergence

[1] Liu C, Zhang M, Yan X, Ni Y, Gong Y, Wang C, et al. (2023) Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv. 9(4):eadd8977. https://doi.org/10.1126/sciadv.add8977
[2] Zhang Y, Chen C, Liu Z, Guo H, Lu W, Hu W, et al. (2022) PABPC1-induced stabilization of IFI27 mRNA promotes angiogenesis and malignant progression in esophageal squamous cell carcinoma through exosomal miRNA-21-5p. J Exp Clin Cancer Res. 41(1):111. https://doi.org/10.1186/s13046-022-02339-9
[3] Chu T, Wang Z, Pe'er D, Danko CG. (2022). Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat Cancer. 3(4):505-517. https://doi.org/10.1038/s43018-022-00356-3
[4] Sekiguchi S, Yorozu A, Okazaki F, Niinuma T, Takasawa A, Yamamoto E, et al. (2023) ACLP Activates Cancer-Associated Fibroblasts and Inhibits CD8+ T-Cell Infiltration in Oral Squamous Cell Carcinoma. Cancers (Basel). 15(17):4303. https://doi.org/10.3390/cancers15174303
[5] Wei WF, Zhou HL, Chen PY, Huang XL, Huang L, Liang LJ, et al. (2023) Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells. J Exp Clin Cancer Res. 42(1):160. https://doi.org/10.1186/s13046-023-02714-0
[6] Dumitru CS, Ceausu AR, Gaje NP, Suciu CS, Raica M. (2022). Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int J Mol Sci, 23(17):9793. https://doi.org/10.3390/ijms23179793
[7] Dai J, Xi X, Liu Z, Wu W, Zhu S, Zhang X, et al. (2023) Single-cell sequencing of multi-region resolves geospatial architecture and therapeutic target of endothelial cells in esophageal squamous cell carcinoma. Clin Transl Med. 13(11):e1487. https://doi.org/10.1002/ctm2.1487
[8] Long SY, Shang L, Shi H, Zhao S, Cao J, He Y. (2023). The Future Landscape of Endothelial Cells Research in Psoriasis: Bibliometric Analysis and Literature Review. Clin Cosmet Investig Dermatol. 16(1):3107-3120. https://doi.org/10.2147/CCID.S435085
[9] Hanahan D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell. 144(5):646-74. https://doi.org/10.1016/j.cell.2011.02.013
[10] Li X, Wang C, Zhang H, Li Y, Hou D, Liu D, et al. (2023) circFNDC3B Accelerates Vasculature Formation and Metastasis in Oral Squamous Cell Carcinoma. Cancer Res. 83(9):1459-1475. https://doi.org/10.1158/0008-5472.CAN-22-2585
[11] Hakuno SK, Janson SGT, Trietsch MD, de Graaf M, de Jonge-Muller E, Crobach S, et al. (2023) Endoglin and squamous cell carcinomas. Front Med (Lausanne). 10(1):1112573. https://doi.org/10.3389/fmed.2023.1112573
[12] Su NW, Dai SH, Hsu K, Chang KM, Ko CC, Kao CW, et al. (2024) PD-L1-positive circulating endothelial progenitor cells associated with immune response to PD-1 blockade in patients with head and neck squamous cell carcinoma. Cancer Immunol Immunother. 73(1):3. https://doi.org/10.1007/s00262-023-03595-0
[13] Noh JJ, Kim MS, Cho YJ, Jeong SY, Lee YY, Ryu JY, et al. (2020) Anti-Cancer Activity of As4O6 and its Efficacy in a Series of Patient-Derived Xenografts for Human Cervical Cancer. Pharmaceutics. 12(10):987. https://doi.org/10.3390/pharmaceutics12100987
[14] Shimomura H, Sasahira T, Nakashima C, Kurihara-Shimomura M, Kirita T. (2019). Non-SMC Condensin I Complex Subunit H (NCAPH) Is Associated with Lymphangiogenesis and Drug Resistance in Oral Squamous Cell Carcinoma. J Clin Med. 9(1):72. https://doi.org/10.3390/jcm9010072
[15] Li C, Guan R, Li W, Wei D, Cao S, Xu C, et al. (2023) Single-cell RNA sequencing reveals tumor immune microenvironment in human hypopharygeal squamous cell carcinoma and lymphatic metastasis. Front Immunol. 14(1):1168191. https://doi.org/10.3389/fimmu.2023.1168191
[16] Zhang J, Lu T, Lu S, Ma S, Han D, Zhang K, et al. (2022) Single-cell analysis of multiple cancer types reveals differences in endothelial cells between tumors and normal tissues. Comput Struct Biotechnol J. 21(1):665-676. https://doi.org/10.1016/j.csbj.2022.12.049
[17] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[18] Tokozlu B, Yücel ÖÖ, Gültekin SE, Bozdağ LA. (2024). Epithelial mesenchymal transition and cancer stem cell markers in oral epithelial dysplasia and oral squamous cell carcinoma. Pol J Pathol. 75(4):305-314. https://doi.org/10.5114/pjp.2024.145818.
[19] Franz L, Nicolè L, Frigo AC, Ottaviano G, Gaudioso P, Saccardo T, et al. (2021) Epithelial-to-Mesenchymal Transition and Neoangiogenesis in Laryngeal Squamous Cell Carcinoma. Cancers (Basel). 13(13):3339. https://doi.org/10.3390/cancers13133339
[20] Xiao J, Song Y, Gao R, You M, Deng C, Tan G, et al. (2023) Changes of immune microenvironment in head and neck squamous cell carcinoma in 3D-4-culture compared to 2D-4-culture. J Transl Med. 21(1):771. https://doi.org/10.1186/s12967-023-04650-1
[21] He Z, Tian W, Wei Q, Xu J. (2022). Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front Immunol. 13(1):968649. https://doi.org/10.3389/fimmu.2022.968649
[22] Chen P, Wang Y, Li J, Bo X, Wang J, Nan L, et al. (2021) Diversity and intratumoral heterogeneity in human gallbladder cancer progression revealed by single-cell RNA sequencing. Clin Transl Med. 11(6):e462. https://doi.org/10.1002/ctm2.462
[23] Sun Q, Zhang T, Xiao Q, Mei B, Zhang X. (2022) Procyanidin B2 inhibits angiogenesis and cell growth in oral squamous cell carcinoma cells through the vascular endothelial growth factor (VEGF)/VEGF receptor 2 (VEGFR2) pathway. Bioengineered. 13(3): 6500-6508. https://doi.org/10.1080/21655979.2022.2033013
[24] Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. (2019) KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet, 394(10212): 1915-1928. https://doi.org/10.1016/S0140-6736(19)32591-7
[25] Ozer M, Sahin I . (2022). Nivolumab in Esophageal Squamous-Cell Carcinoma. N Engl J Med, 386(20):1958-1959. https://doi.org/10.1056/NEJMc2202880
Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Life Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2024-10-17
Accepted 2024-12-20
Published 2024-12-30


