Abstract

Enhancer of zeste homolog 2 (EZH2), a core member of the Polycomb Group (PcG) family, is a pivotal
epigenetic regulator. As the catalytic subunit of Polycomb Repressive Complex 2 (PRC2), EZH2 mediates
trimethylation of histone H3 lysine 27 (H3K27me3), leading to chromatin condensation and altered
expression of downstream genes. This mechanism enables EZH2 to exert multidimensional roles in
development, tumors, immunity, and the nervous system. Given its critical role in epigenetic
regulation and multidimensional oncogenesis, EZH2 has emerged as a hot target for cancer therapy. This
review summarizes EZH2's regulatory functions and specific pro-tumorigenic mechanisms, detailing its
roles in epigenetic regulation, tumor proliferation and metastasis, tumor microenvironment, stemness
maintenance, drug resistance, metabolic reprogramming, and dysregulated signaling pathways, aiming to
inspire new perspectives in cancer treatment research.
Keywords: EZH2, epigenetic modification, methylation, H3K27me3, cancer
Background
Cancer occurrence is highly correlated not only with genetics, diet, infection, microbiota, smoking, alcohol
consumption, and other factors [1-4], but also with epigenetic dysregulation (such as DNA methylation, histone
modifications, and non-coding RNA regulation), which silences tumor suppressor genes and activates oncogenes, thereby
playing a key role in driving cancer formation [5-7]. Histone modification, as one of the core mechanisms in
epigenetics for regulating gene expression, alters chromatin structure through chemical modifications, thereby
affecting DNA accessibility and gene transcriptional activity [3, 8]. Among these, trimethylation of histone H3 lysine
27 (H3K27me3), catalyzed by Polycomb Repressive Complex 2 (PRC2), is a key type of histone modification and serves as
a critical repressive mark in epigenetic regulation [9, 10].
Enhancer of zeste homolog 2 (EZH2), as the core subunit of PRC2, exerts its oncogenic effects mainly via two modes:
the PRC2-dependent classical function and the PRC2-independent non-classical function (Figure 1). The classical
function involves EZH2 participating in the formation of the PRC2 complex and binding to the promoter regions of
target genes, where it catalyzes H3K27me3 to create a condensed chromatin structure, thereby hindering the binding of
RNA polymerase II and transcription factors and affecting downstream gene expression [11, 12]. The non-classical
function involves EZH2 directly methylating non-histone proteins, resulting in downstream gene activation or
ubiquitin-mediated degradation without the need to form the PRC2 complex [13, 14]. However, recent studies have shown
that EZH2 can even function independently of its methyltransferase activity by acting as a transcriptional
co-activator that directly binds to target proteins to activate downstream targets, or by directly binding to the
promoters of metabolic genes to regulate gene activation [15-17]; thus, these processes are not blocked by enzymatic
EZH2 inhibitors.
Epigenetic Regulation of EZH2 in Tumors
EZH2 Catalyzes Methylation of Histone
Histone methylation, as the most central catalytic function of EZH2,
drives gene silencing by mediating H3K27 trimethylation, thereby suppressing the expression of tumor suppressor genes
and promoting cell cycle progression. In a controlled RT-PCR study comparing cholangiocarcinoma tissues with or
without EZH2 depletion, it was found that EZH2 knockdown led to significant upregulation of 12 key tumor suppressor
genes (such as PAX3, SFRP1, CDKN1A, and GAS1), suggesting that EZH2 may promote cholangiocarcinoma cell growth through
epigenetic silencing of tumor suppressor genes [11]. Overexpression of miR-139-5p can induce cellular senescence and
inhibit hepatocellular carcinoma cell proliferation. EZH2 promotes TOP2A expression by epigenetically silencing
miR-139-5p via H3K27me3, thereby driving hepatocellular carcinoma cell proliferation and malignant progression [12].
Regarding dysregulated cell cycle control in cancer cells, a study found that inhibition of EZH2 expression in
multiple myeloma cells increased the levels of cyclin-dependent kinase inhibitors p15 and p21, and this upregulation
was associated with the CDKN2B and IFIT3/MYC/CDKN1A pathways [18]. Pan-cancer analyses have shown that PRC2 is
enriched at the promoter regions of EMT-related genes, directly suppressing the expression of epithelial markers such
as E-cadherin [19-21].
Non-Coding RNAs Regulates EZH2 Stability
Studies have revealed that in hepatocellular carcinoma cells,
non-coding RNA such as miR-26a, miR-138-5p, and miR-144/451a form a negative feedback loop with EZH2 to regulate
cancer cell growth: EZH2-mediated H3K27me3 modification at the promoter regions suppresses the transcription of these
miRNAs, while luciferase reporter assays demonstrate that these miRNAs directly target the 3'UTR of EZH2 mRNA,
directing post-transcriptional repression and thus modulating gene expression [22-24]. FUS protein is a
multifunctional nucleic acid-binding protein that can recognize and bind specific sequence elements within the 3'UTR
of target mRNA in cells, thereby preventing the binding of other factors that promote mRNA degradation or structurally
enhancing mRNA stability; it thus exerts important biological functions [25, 26]. Non-coding RNA such as
hsa_circ_0006232, LINC00313, and LINC SNHG14 have been found to promote the interaction between FUS protein and EZH2,
prolong the half-life of EZH2 mRNA, increase its stability, and consequently enhance EZH2-mediated epigenetic
silencing of downstream factors (e.g., PTEN and EPHA7), thereby facilitating tumor progression [27-29].
Dynamic Assembly of PRC2 Complex
EZH2 Y641 mutation promotes multiround H3K27 methylation by altering
H-bonding patterns and/or steric crowding within the enzyme–bisubstrate ternary complex active site; in other words,
the Y641-mutant EZH2 changes substrate preference, preferentially catalyzing the conversion of H3K27me2 to H3K27me3,
leading to the formation of lymphoma-specific H3K27me3 hyperdomains [30, 31]. Mutations in SUZ12 and EED are highly
conserved; among them, the Suz12 D605V and Eed G255D mutations markedly reduce the histone methyltransferase activity
of the PRC2 complex and impair the catalytic formation of H3K27me3 [32].
The Role of EZH2 in Tumor Proliferation,Invasion and Metastasis
EZH2-Related Signaling Axes and Non-Coding RNA Regulatory Networks Influence EMT Process
Epithelial-mesenchymal transition (EMT) in tumor cells is crucial for promoting metastasis and invasion, and this
process can be regulated by multiple signaling axes, such as EZH2-MIR-293A-SNAI [33], Snail/Slug-MIR-101-EZH2 [34],
MIR-217-MALAT1-EZH2-H3K27me3 [35], and TGF-β-MTA1-SMAD7-SMAD3-SOX4-EZH2 [36]. Long non-coding RNA can recruit EZH2 to
exert targeted repression of epithelial markers, thereby facilitating EMT; for example, LINC01133 brings EZH2 and LSD1
to the promoters of KLF2, P21 and E-cadherin to silence their transcription and enhance tumor invasion and metastasis
[37]; LINC00978 recruits EZH2 to the promoters of P21 and E-cadherin to promote EMT [19]. In addition to recruitment,
LINC00152 can release EZH2 after binding to the PRC2 complex, thereby reducing H3K27me3 enrichment at the ZEB1
promoter and blocking its transcriptional repression, which promotes EMT and oxaliplatin resistance in esophageal
cancer cells [38].
Non-Canonical Pathways Promote Tumor Invasion and Metastasis
In addition to epigenetic regulation of
histones, splicing-dependent mechanisms also play important roles in cancer cell EMT. Studies have shown that
LINC01348 competitively binds to splicing factor 3B subunit 3 (SF3B3), blocking efficient splicing of EZH2 pre-mRNA
and leading to loss of functional EZH2 protein, thereby inhibiting hepatocellular carcinoma invasion [39]. Moreover,
the EZH2-catalyzed product H3K27me3 has been found to act as an atypical repressor driving peritoneal metastasis in
triple-negative breast cancer. Specifically, H3K27me3 is markedly enriched at the promoter of the KRT14 gene; it
compresses the GC-box region within the KRT14 promoter to prevent binding of the repressor SP1 and permits RNA
polymerase II to initiate transcription of KRT14, thereby promoting triple-negative breast cancer migration, invasion,
and peritoneal metastasis. In this study, enrichment of H3K27me3 at the KRT14 promoter did not repress but instead
enhanced its transcription [40]. In addition, post-translational modifications of non-histone proteins—such as
phosphorylation, methylation, acetylation, and ubiquitination—closely influence protein stability, activity
modulation, and protein–protein interactions [3, 41-45]. It has been reported that EZH2, acting as a
methyltransferase, directly catalyzes trimethylation of lysines K53 and K333 on SMAD3 under stimulation by the tumor
suppressor TGF-β; subsequently, methylated SMAD3 modulates SMAD3 S423/S425 phosphorylation through regulated membrane
recruitment, thereby promoting tumor metastasis [46].
EZH2-Regulated Tumor Proliferation and Metastasis via Wnt/β-catenin Signaling Pathway
According to the FpClass database, EZH2 can interact with metal-response element-binding transcription factor 2
(MTF2), and the Wnt pathway antagonist secreted frizzled-related protein 1 (SFRP1) has been identified as a target
gene of EZH2. Subsequent studies demonstrated that MTF2 promotes EMT progression in osteosarcoma via the
EZH2/SFRP1/Wnt signaling axis [47, 48]. Beyond osteosarcoma, EZH2-mediated regulation of the Wnt/β-catenin pathway in
tumor proliferation and metastasis has been validated in multiple cancer types, including colorectal cancer [49, 50],
gastric cancer [51], melanoma [52, 53], glioma [54], laryngeal cancer [55], cervical cancer [56], and liver cancer
[57], establishing a pan-cancer theoretical and practical basis for EZH2-targeted therapy to suppress the
Wnt/β-catenin pathway driving malignant progression. Nevertheless, the precise mechanisms may vary according to tumor
heterogeneity and tissue specificity.
Tumor Microenvironment Remodeling
Studies have found that EZH2 deletion or inhibition causes opposite CCL2 expression and thus influences different
polarization states of tumor-associated macrophages (TAMs). Whether this depends on histone methyltransferase activity
can explain the up-regulation: after treatment with the EZH2 inhibitor EPZ-6438, increased CCL2 levels lead to
elevated M2-type TAMs and higher tumor vascular density, further promoting tumor metastasis. Meanwhile, EZH2 can
recruit DNMT1 to the miR-124-3p promoter that targets CCL2, forming a hypermethylated structure; this explains why
EZH2 loss results in CCL2 down-regulation [58]. Therefore, the above studies indicate that EZH2 exerts opposite
regulatory effects on TAMs polarization in breast cancer through its enzymatic or non-enzymatic activities. In
addition, contradictory findings show that EZH2 inhibitors can reprogram tumor cells into a more immunogenic state.
Specifically, EZH2 inhibitors up-regulate genes related to adhesion, inflammatory response, and B-cell activation in
the tumor microenvironment, thereby inhibiting tumor progression and metastasis [59, 60]. From these studies we can
see that EZH2-mediated regulation of the tumor immune microenvironment involves highly complex mechanisms with dual
roles, reminding us to be especially cautious when choosing EZH2 inhibitors in different tumor microenvironments;
otherwise, not only may the expected therapeutic effect not be achieved, but tumor progression may even be
exacerbated. Beyond differences in immune cell types and distribution, the hypoxic tumor microenvironment is also a
major challenge in solid tumor therapy; in hepatocellular carcinoma cells, hypoxia-induced elevation of LINC00839 is
closely associated with a series of malignant phenotypes [61]. Related studies have shown that under hypoxic
conditions EZH2 expression and activity increase, thereby affecting tumor cell proliferation, invasion, and
metastasis. Moreover, EZH2 can actively participate in remodeling the tumor microenvironment by regulating
hypoxia-related factors such as HIF-1α, promoting tumor EMT and malignant progression [62–64].
Roles of EZH2 in Tumor Drug Resistance
EZH2 Affects Drug Resistance through Classical Pathways
In ovarian cancer, c-Myc enhances EZH2 expression
by directly repressing miR-137, which targets EZH2 mRNA; conversely, EZH2 can silence miR-137, further strengthening
the negative-feedback loop that modulates tumor resistance to cisplatin [65]. Similarly, in esophageal squamous
carcinoma cells, CTCF recruits EZH2 and PXN proteins to the miR-137 promoter to inhibit its expression, while EZH2 and
PXN mRNAs are downstream targets of miR-137, thereby regulating cellular radiosensitivity through this
negative-feedback mechanism [66]. In non-small-cell lung cancer, EZH2 catalyzes H3K27me3 at the puma promoter to
silence the apoptosis regulator puma and influence sensitivity to platinum-based agents [67]. In glioblastoma, a
circular EZH2-encoded EZH2-92aa protein suppresses NKG2D-ligand expression, allowing tumor cells to evade NK-cell
cytotoxicity; although no immunotherapeutics were directly tested, this finding suggests that cellular immunotherapy,
immune-checkpoint inhibitors, and cytokine-based drugs (e.g., IL-2, IL-15) may be less effective in glioblastoma [68].
In chronic myeloid leukemia, the lncRNA HOTTIP recruits EZH2 to the PTEN promoter, causing hypermethylation and PTEN
down-regulation, which induces resistance to imatinib mesylate [69]. Collectively, these studies demonstrate that
EZH2-mediated epigenetic resistance mechanisms critically influence resistance to traditional platinum cytotoxics,
immunotherapeutics, radiotherapy, and small-molecule targeted agents across both solid and hematologic malignancies,
underscoring EZH2’s broad significance in tumor drug resistance. While the precise mechanisms and hierarchical roles
of EZH2 in resistance remain incompletely understood, combining EZH2 inhibitors with anticancer drugs holds promise
for enhancing drug sensitivity and reducing resistance. To date, such combination studies are largely confined to
preclinical models and have shown improved therapeutic outcomes; however, corresponding clinical data are scarce,
highlighting the need for expanded translational and clinical investigations.
EZH2 Affects Drug Resistance through Non-Classical Pathways
BRAF mutation activation is the most frequent
alteration in cutaneous malignant melanoma [70–72], and BRAF-targeted agents have been highly anticipated for melanoma
therapy. Nevertheless, clinical data show that approximately 50% of patients develop varying degrees of resistance
after 6–8 months of BRAF-inhibitor treatment [73]. One study found that adding the EZH2 inhibitor EPZ-6438 to
vemurafenib markedly improves the response in BRAF-resistant melanoma cells (A375R), as evidenced by decreased
viability, cell-cycle arrest, and increased apoptosis. The authors hypothesized that EZH2 may act as a transcriptional
co-activator that stimulates transcription of the proto-oncogene PLK1 by associating with E2F1, thereby enhancing PLK1
expression. An alternative mechanism proposes that EZH2 directly methylates STAT3, increasing its phosphorylation;
STAT3 then functions as a PLK1 transcriptional activator [74], ultimately leading to PLK1 overexpression [75].
However, several questions surround these mechanistic assumptions. First, if EZH2 indeed serves as a transcriptional
co-activator that partners with E2F1 to drive PLK1 transcription, its effect should not be sensitive to
methyltransferase inhibitors. Second, the study observed that EZH2 inhibition alone had no impact on PLK1 expression
in melanoma cells, indicating that EZH2 inhibitors merely act synergistically with vemurafenib and do not directly
suppress PLK1—an outcome that contradicts the proposed mechanism. Therefore, more complex regulatory networks likely
remain to be uncovered.
EZH2-Regulated Tumor Drug Resistance via PI3K/AKT Pathway
The PI3K/AKT pathway is the most frequently
reported signaling axis through which EZH2 influences tumor drug resistance. In most studies EZH2 acts as an activator
of this pathway; however, owing to tissue- and tumor-specific contexts, the exact activation modes differ across
cancer types. In acute myeloid leukemia, combined use of the EZH2 inhibitor DZNep and the selective BCL-2 inhibitor
venetoclax achieved synergistic efficacy and markedly increased venetoclax sensitivity. Whole-transcriptome analysis
revealed that PIK3IP1 (a negative regulator of PI3K/AKT/mTOR signaling) is inversely correlated with EZH2 expression,
indicating that EZH2 epigenetically up-regulates the PI3K/AKT pathway to mediate cellular resistance [76]. In chronic
myeloid leukemia, LINC-HOTTIP recruits EZH2 to the PTEN promoter, inducing hypermethylation and down-regulating PTEN,
thereby driving imatinib resistance [69]. Although the study did not explicitly address PI3K/AKT activation, PTEN is a
well-established lipid phosphatase that dephosphorylates PIP3 to PIP2 and suppresses AKT activity across numerous
cancers [69, 77, 78]. While EZH2 generally functions as a PI3K/AKT activator, it can also inhibit this pathway in
specific settings. In colorectal cancer, LINC-PiHL binds EZH2 and reduces its occupancy at the HMGA2 promoter,
lowering H3K27me3 levels at this locus. Up-regulated HMGA2 then enhances PI3K/AKT phosphorylation, leading to
oxaliplatin resistance [79]; here, EZH2 behaves as a PI3K/AKT inhibitor. Collectively, these findings illustrate
EZH2’s multi-target, cell-type, and microenvironment-dependent functions.
Stemness Maintenance
A large body of research has confirmed that EZH2 can act as a cancer stem cell marker and is highly expressed in
neuroblastoma, epidermal carcinoma, high-risk cytomegalovirus-infected breast cancer, medulloblastoma, neural stem
cells, pancreatic cancer, and other tumor types, thereby promoting tumor self-renewal and metastatic capacity [80-86].
Correspondingly, depletion of EZH2 suppresses the self-renewal ability of tumor cells and reduces their proliferation
and invasive potential [87-89].
Non-coding RNA Regulatory Networks
Similarly, EZH2 sustains tumor cell stemness through multiple
mechanisms. Most commonly, lncRNA recruits EZH2 to the promoters of tumor-suppressor genes to catalyze H3K27me3
formation, thereby promoting CRC cell proliferation or stemness maintenance. For example, LINC01419 recruits EZH2 to
the FBP1 promoter to enhance lung adenocarcinoma cell proliferation and stemness [90]; LINC-HOXB-AS3 recruits EZH2 to
the Dicer promoter, epigenetically repressing its transcription to foster sorafenib resistance and stemness
maintenance in hepatocellular carcinoma cells [91]. Beyond recruitment, lncRNA can also serve as molecular “sponges”
to influence EZH2 expression. LINC-PDZD7, for instance, acts as a miR-101 sponge, preventing the latter from binding
to EZH2 mRNA, thereby increasing EZH2 levels and suppressing the stemness regulator ATOH8 [92]. Intuitively, stronger
tumor stemness should correlate with stronger immune evasion, and studies have already shown that certain lncRNAs are
closely linked to tumor immune features [93]. Therefore, it is reasonable to hypothesize that lncRNA may modulate
tumor stemness via EZH2, in turn affecting immune responsiveness—a mechanism that clearly warrants further
investigation.
Synergistic DNA Methylation Effect
Co-immunoprecipitation revealed that TRIM37 interacts with EZH2 to
maintain glioma cell growth and stemness; the underlying mechanism involves down-regulation of the Sonic Hedgehog
pathway inhibitor PTCH1, thereby activating the Sonic Hedgehog stem cell signaling pathway [94].
Glycolysis
Tumor cells reprogram metabolism to increase glucose uptake and accelerate glycolysis,
producing energy mainly via glycolysis even under abundant oxygen—a phenomenon termed the “Warburg Effect” [95, 96].
Although this mode is energetically inefficient, it supplies proliferating cells with metabolic intermediates.
Numerous studies have confirmed the close relationship between EZH2 and tumor metabolic abnormalities [97]. In
glioblastoma, an EZH2-EAF2-pVHL-HIF-1α axis drives glycolysis and malignant progression: EAF2 binds and stabilizes
pVHL (a tumor suppressor that mediates degradation of the glycolysis master regulator HIF-1α [98, 99]), whereas EZH2
epigenetically silences EAF2, elevating HIF-1α and reprogramming metabolism [100]. Additionally, the
EZH2-miR-328-β-catenin axis has been shown to promote glioma glycolysis, indicating that EZH2 can modulate glycolysis
via signaling pathways [101]. Intriguingly, in hypoxia-induced HIF-1α-upregulated non-small-cell lung cancer models,
EZH2 expression is also increased; EZH2 then epigenetically represses FBXL7, reducing ubiquitin-mediated degradation
of its substrate PFKFB4 and ultimately enhancing glucose metabolism and malignant phenotypes [102]. Given the
cooperative, complementary oncogenic roles of HIF-1α and EZH2 in diverse tumors, the dual-target compound DYB-03 was
screened and found to induce stronger apoptosis and angiogenesis inhibition in lung cancer cells than single-target
inhibitors [103]. In bladder cancer, aldehyde oxidase 1 (AOX1) suppresses the tryptophan-kynurenine pathway, lowering
NADP levels and decreasing pentose-phosphate-pathway flux and nucleotide synthesis, thereby weakening tumor invasion;
EZH2-mediated epigenetic silencing of AOX1 reverses this, increasing pentose-phosphate-pathway flux and nucleotide
synthesis [104]. In colorectal cancer, the homeobox transcription factor PROX1 recruits EZH2 to the SIRT3 promoter,
repressing SIRT3 and promoting tumor cell proliferation and glycolysis [105]. Non-canonical actions have also been
described: in ovarian malignancies, cell-proliferation assays and immunoblotting after treatment with EZH2 inhibitor
(DZNep), EZH2 degrader (YM281), or EZH2 catalytic inhibitors (GSK126, EPZ-6438) revealed that EZH2’s oncogenic role is
independent of its catalytic activity. Integrated ChIP-seq and RNA-seq data showed EZH2 directly binds and
transcriptionally activates metabolic genes related to the TCA cycle and OXPHOS, such as IDH2 and OGDHL, thereby
fostering metabolic adaptation and malignant progression [17]. In summary, EZH2 markedly promotes tumor-cell
glycolysis to drive cancer cell proliferation.
Lipid Metabolism
Rapidly proliferating tumor cells usually enhance fatty acid oxidation (FAO) and
synthesis to satisfy the demand for membrane and signaling-molecule production [106]; yet EZH2’s influence on tumor
lipid levels exhibits contradictory, context-dependent effects across species and organs [107-110]. Thus, a
comprehensive dissection of EZH2’s specific lipid-metabolic targets and signaling axes—together with cell-type
specificity—is urgently needed for precise clinical guidance. In triple-negative breast cancer, EZH2 interacts with
the metabolic key enzyme PKM2 to form an epigenetic-regulatory complex that reprograms cellular metabolism from
glycolysis toward FAO-β-oxidation, thereby supporting tumor growth [111]. In ARMH1-knockdown leukemia cells (MOLM-14
and HEL92.1.7), supplementation of exogenous lipid mixtures restored proliferation to levels comparable with wild-type
controls, indicating ARMH1’s involvement in leukocyte fatty acid (FA) synthesis. Subsequent CO-IP confirmed a physical
interaction between ARMH1 and EZH2, suggesting functional interdependence linked to lipid metabolism [112]. Moreover,
oHSV-infected glioblastoma cells exhibited elevated EZH2 expression and a pronounced increase in FAO, implying that
EZH2 plays a key role in lipid-metabolic reprogramming in these tumors [113]. Notably, high intratumoral FA levels not
only sustain energy supply and membrane synthesis but also broadly impact the anti-tumor efficacy of EZH2 inhibitors
(GSK126, EPZ-6438): EZH2i reduces H3K27me3 at the promoters of lipid-synthesis genes SCD1 and ELOVL2, leading to
elevated FA levels and attenuated suppression of melanoma growth. Combining EZH2i with lipid-lowering agents markedly
enhanced tumor-growth inhibition, demonstrating that EZH2i profoundly influences tumor lipid metabolism and that lipid
levels in turn modulate cellular sensitivity to EZH2i [114]. Accordingly, recent studies on targeted disruption of
tumor oxidative defense further confirmed that various EZH2 inhibitors induce dysregulation of lipid-metabolic genes
[115].
Amino Acid Metabolism
EZH2 expression in colorectal cancer is inversely correlated with glutaminase
levels; thus, EZH2 down-regulation accelerates glutamine hydrolysis to glutamate, increasing glutathione synthesis and
enhancing cellular antioxidant capacity, thereby attenuating ROS-induced cell death under glucose deprivation—a
mechanism potentially linked to tolerance to EZH2 inhibitors [116]. EZH2 influences amino-acid metabolism via two
principal routes:(1) suppressing glutamine metabolism as described above,(2) promoting S-adenosyl-methionine (SAM)
synthesis. Studies show EZH2 orchestrates a cascade that regulates methionine availability: EZH2 binds the retinoid X
receptor α (RXRα) promoter to repress its transcription; diminished RXRα relieves inhibition of LAT1, whose
up-regulation then increases methionine import. Elevated methionine satisfies tumor metabolic demand and serves as a
methyl donor for DNA and histone methylation [117, 118]. In lung adenocarcinoma, EZH2 inhibition activates
Gamma-Aminobutyric Acid (GABA) synthesis because the GAD1 and GAD2 genes are epigenetically silenced by H3K27me3;
GABA, a key neurotransmitter, may modulate tumor–stromal cell communication within the tumor microenvironment [119].
Although amino acids act as carbon and nitrogen sources, fueling rapid proliferation and macromolecule synthesis, and
their metabolites can act as signaling molecules to modulate oncogenic pathways, the underlying regulatory mechanisms
remain poorly understood. Thus, elucidating how EZH2, already implicated in amino-acid metabolism, orchestrates
metabolic reprogramming is of paramount importance (Figure 2).
Signaling Pathways Dysregulation
PI3K/AKT/mTOR Signaling Pathway
The PI3K/AKT/mTOR pathway is a key intracellular signaling axis that
widely participates in proliferation, survival, differentiation, angiogenesis, drug resistance, and other biological
processes [120–122]. In cancer, activation of this pathway is closely associated with tumor metastasis, invasion, and
resistance [123–125]. Among all signaling cascades, the PI3K/AKT/mTOR pathway is the most tightly linked to EZH2: in
multiple cancer types, EZH2 is recruited or bound by other biomarkers to epigenetically silence the PI3K/AKT inhibitor
PTEN, thereby activating the pathway and influencing cancer-cell proliferation, stemness maintenance, metastasis,
invasion, and drug resistance—examples include osteosarcoma [27], leukemia [69], colorectal cancer [126], gastric
cancer [127], and prostate cancer [128]. Interestingly, activation of PI3K/AKT can also directly or indirectly mediate
phosphorylation of EZH2 at S21; for instance, in ablation cells, PI3K/AKT activity positively correlates with EZH2-S21
phosphorylation. Phosphorylation at S21 is precisely the switch that converts EZH2 from a Polycomb-dependent
transcriptional repressor into a transcriptional co-activator that cooperates with factors such as the androgen
receptor to activate downstream gene expression [129].
Wnt/β-catenin Signaling Pathway
It is well known that the Wnt/β-catenin pathway plays essential roles in
embryonic development [130], cell proliferation, differentiation, and tissue homeostasis. In cancer, this pathway
markedly promotes excessive extracellular-matrix degradation, tumor invasion and migration, drug resistance, and
stemness maintenance [131]. In normal esophageal epithelial cells, EZH2 binds the WNT2 promoter and, via its histone
methyltransferase activity, represses WNT2 expression, preventing aberrant Wnt activation. In contrast, esophageal
squamous-cell carcinoma shows reduced EZH2 occupancy at the WNT2 promoter, relieving repression and increasing WNT2
transcription [132]. Similar mechanisms operate in glioblastoma, bladder cancer, and hepatocellular carcinoma, where
LINC-H19 recruits EZH2 to the promoters of Wnt inhibitors AXIN2 and NKD1, depositing H3K27me3 and thereby
up-regulating Wnt/β-catenin signaling [133, 134]. In glioblastoma, HP1 recruits histone deacetylases (HDACs) to
H3K9me2-marked loci to silence gene transcription, but EZH2–HP1BP3 interaction impairs HP1-mediated HDACs recruitment,
reducing H3K9me2 and derepressing WNT7B, which influences temozolomide resistance [135]. In colorectal cancer, PAF—an
overexpressed translesion DNA-synthesis component—dissociates from PCNA upon Wnt activation, binds β-catenin directly,
and recruits EZH2 to form a transcriptional activation complex that boosts β-catenin target gene expression,
independent of EZH2 methyltransferase activity [136]. Collectively, EZH2 up- or down-regulates Wnt/β-catenin signaling
in different tumors via both canonical and non-canonical mechanisms, thereby influencing proliferation, metastasis,
drug resistance, and other malignant phenotypes. The precise mode of action depends on tumor type, cellular
microenvironment, and pathway crosstalk.
STAT Signaling Pathway
Studies have shown that EZH2 can exert non-canonical functions by directly binding
to and methylating the STAT3. After methylation, STAT3 undergoes further phosphorylation at the Y705 site, leading to
enhanced transcriptional activity—clearly a direct phosphorylation process mediated by EZH2 on STAT3. In addition to
directly phosphorylating STAT3, EZH2 can also indirectly phosphorylate the STAT signaling pathway by regulating JAK2.
The STAT3 pathway is widely upregulated in various cancers and is closely associated with malignant phenotypes such as
tumor cell apoptosis, invasion, and drug resistance, including in glioblastoma [13, 137], melanoma [75], renal cancer
[138], breast cancer [139], and colorectal cancer [140]. Moreover, it has been found that EZH2-mediated regulation of
the STAT signaling pathway is involved in the glycolytic process of oral squamous cell carcinoma, providing energy and
substrates for rapidly growing tumor cells and facilitating the EMT process [141–143], as well as in regulating the
polarization of M1-type macrophages toward the M2 phenotype [144, 145]. Furthermore, after chemotherapy treatment, the
EZH2/STAT3 pathway in breast cancer cells is activated, which in turn stimulates the secretion of exosomes carrying
miR-378a-3p and miR-378d targeting DKK3 and NUMB, ultimately upregulating the Wnt/β-catenin and Notch pathways to
promote tumor drug resistance and stemness maintenance [146]. In summary, the EZH2-regulated STAT signaling pathway
plays multiple critical roles in tumor development, metabolic reprogramming, immune microenvironment modulation,
invasion, metastasis, and resistance formation. This suggests great potential for the future development of
combination therapies using EZH2 inhibitors/degraders and STAT3 inhibitors. However, the complex crosstalk mechanisms
within these pathways also indicate that targeting crosstalk nodes may be more effective than inhibiting a single
pathway alone.
ERK Signaling Pathway
Studies have shown that the MEK–ERK–Elk-1 signaling axis promotes EZH2
overexpression in triple-negative and ERBB2-overexpressing aggressive breast cancer subtypes by modulating Elk-1
binding sites within the EZH2 promoter. Using promoter assays, cellular experiments, and tissue analyses, the research
team demonstrated that MEK inhibitors and Elk-1 siRNA markedly reduce EZH2 mRNA and protein levels. In this context,
EZH2 functions as a downstream target gene of the MEK/ERK pathway, influencing breast cancer cell proliferation and
invasion [147]. Meanwhile, EZH2 knockdown was found to down-regulate the AKT/ERK signaling cascade, thereby
suppressing FSH secretion and inhibiting the development of ovarian cancer in sheep [148]. Additionally, EZH2-mediated
silencing of the tumor-suppressor gene SMAD4 activates the ERK/c-Myc pathway, driving resistance in non-small-cell
lung cancer [149].
YAP Signaling Pathway
YAP functions as either an oncogene or a tumor suppressor in various cancers.
Studies show that EZH2 can form a complex with YAP and the multifunctional transcription factor YY1, facilitating
epigenetic modification of the cyclin-dependent kinase inhibitor P27 promoter region and thereby promoting cell
proliferation [150]. Similarly, EZH2, together with MYC and YAP/TAZ proteins, can assemble a transcriptional repressor
complex that down-regulates the tumor suppressor PTEN [151]. Beyond these transcriptional repressor complexes, the
non-canonical Wnt pathway is also critical for maintaining YAP protein levels [152] (Figure 3).
Other Pathways
In addition to the major signaling cascades described above, EZH2 participates in the
regulation of several non-dominant yet important pathways. For example, in breast cancer the long isoform of NSD3
(NSD3-long) cooperates with EZH2 and RNA polymerase II to stimulate transcription of genes involved in NOTCH receptor
cleavage, leading to nuclear accumulation of NICD1. Overexpressed NICD1 then mediates transcriptional repression of
E-cadherin and promotes tumor EMT [153]. Other studies have demonstrated that EZH2 can elevate H3K27me3 levels at the
GADD45A promoter to suppress its transcription; reduced GADD45A expression subsequently activates the p38/MAPK pathway
[154].
Conclusion
EZH2, a pivotal epigenetic regulator, exerts complex and multidimensional roles in tumorigenesis, progression,
metastasis, and drug resistance. Via the canonical PRC2 complex it catalyzes H3K27me3 to silence tumor-suppressor
genes, thereby promoting proliferation and invasion, whereas non-canonical actions—direct methylation of non-histone
proteins or functioning as a transcriptional co-activator—support stemness maintenance, metabolic reprogramming, and
tumor-microenvironment remodeling. During invasion and metastasis, EZH2 drives migration through modulation of
EMT-related axes, interference with splicing, and post-translational modification of non-histone proteins; its
crosstalk with the Wnt/β-catenin pathway has been validated in multiple cancers, demonstrating both pan-cancer
relevance and tissue specificity. In the tumor microenvironment, EZH2 depletion or inhibition can exert opposing
effects on TAMs polarization, and its elevated expression and activity under hypoxia further fuel malignant
progression. Regarding drug resistance, EZH2 participates in epigenetic silencing as well as non-canonical resistance
mechanisms, influencing BRAF-inhibitor resistance and interacting with reprogrammed signaling pathways. As a stemness
marker, EZH2 is highly expressed across tumors, fostering self-renewal and metastasis through non-coding RNA networks,
cooperative DNA methylation, and signaling circuitries. Metabolically, EZH2 markedly enhances glycolysis, alters
fatty-acid synthesis, and modulates amino-acid pathways to supply energy and biosynthetic precursors for rapid
proliferation. Pathway-wise, EZH2 broadly regulates PI3K/AKT/mTOR, Wnt/β-catenin, STAT, ERK, YAP, and others,
impacting proliferation, stemness, invasion, metastasis, and drug resistance. Despite its prevailing oncogenic image,
accumulating evidence indicates context-dependent or even tumor-suppressive roles for EZH2, underscoring its
functional complexity and prompting deeper investigation. Given EZH2’s multifaceted significance, it has become a
prime therapeutic target. Multiple EZH2 inhibitors are in clinical trials and show favorable tolerability and
antitumor activity. However, functional heterogeneity across tumor types and individuals necessitates further
dissection of EZH2 mechanisms within distinct microenvironments, development of precision strategies, and mitigation
of potential resistance. Combining EZH2-targeted agents with immunotherapy, chemotherapy, or other modalities holds
great promise for breakthroughs that could improve patient outcomes and quality of life.
Abbreviations
Aldehyde Oxidase 1: AOX1; Androgen Receptors: AR; Epitheli-al-Mesenchymal Transition: EMT; Enhancer of Zeste
Homolog 2: EZH2; Fatty Acid: FA; Fatty Acid Oxidation: FAO; Gamma-Am-inobutyric Acid: GABA; Histone
Deacetylases: HDACs; Met-al-Response Element-Binding Transcription Factor 2: MTF2;Secreted Frizzled-Related
Protein 1: SFRP1; Trimethylation of Histone H3 Lysine 27: H3K27me3; Polycomb Group: PcG; Polycomb Repressive
Complex 2: PRC2; Retinoid X Receptor α: RXRα; S-Adenosyl-Methionine: SAM; Tumor-Associated Macro-phages:
TAMs.
Declarations
Author contributions
Mingfen Li and Hongsheng Lin were in charge of the proof-reading and design of the manuscript. Hui Yin took
responsi-bility for the writing of the manuscript. Jinna Tan and Jiaqian He were responsible for the
collection and organization of literature. All authors read and approved the final manuscript.
Acknowledgements
Figures in the article were drawn using Biorender: https://www.biorender.com/.
Funding information
This work was supported by Natural Science Foundation of Guangxi Zhuang Autonomous Region
(2025GXNSFDA069035, 2025GXNSFAA069372).
Ethics approval and consent to participate
Not Applicable.
Competing Interests
The authors declare that they have no existing or potential commercial or financial relationships that could
create a con-flict of interest at the time of conducting this study.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary
Materials. Addi-tional data related to this paper may be requested from the authors.
References
[1] WU Z, XIA F, LIN R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980-2021: a systematic analysis for the GBD 2021 [J]. J Hematol Oncol, 2024, 17(1): 119.
[2] KENTSIS A. Why do young people get cancer? [J]. Pediatr Blood Cancer, 2020, 67(7): e28335.
[3] ELANGOVAN A, LI Y, PIRES D E V, DAVIS M J, VERSPOOR K. Large-scale protein-protein post-translational modification extraction with distant supervision and confidence calibrated BioBERT [J]. BMC Bioinformatics, 2022, 23(1): 4.
[4] SEPICH-POORE G D, ZITVOGEL L, STRAUSSMAN R, HASTY J, WARGO J A, KNIGHT R. The microbiome and human cancer [J]. Science, 2021, 371(6536).
[5] NISHIYAMA A, NAKANISHI M. Navigating the DNA methylation landscape of cancer [J]. Trends Genet, 2021, 37(11): 1012-27.
[6] CAO J, YAN Q. Cancer Epigenetics, Tumor Immunity, and Immunotherapy [J]. Trends Cancer, 2020, 6(7): 580-92.
[7] MENON A, ABD-AZIZ N, KHALID K, POH C L, NAIDU R. miRNA: A Promising Therapeutic Target in Cancer [J]. Int J Mol Sci, 2022, 23(19).
[8] TANG J, ZHUANG S. Histone acetylation and DNA methylation in ischemia/reperfusion injury [J]. Clin Sci (Lond), 2019, 133(4): 597-609.
[9] JIANG L, HUANG L, JIANG W. H3K27me3-mediated epigenetic regulation in pluripotency maintenance and lineage differentiation [J]. Cell Insight, 2024, 3(4): 100180.
[10] GLANCY E, WANG C, TUCK E, HEALY E, AMATO S, NEIKES H K, et al. PRC2.1- and PRC2.2-specific accessory proteins drive recruitment of different forms of canonical PRC1 [J]. Mol Cell, 2023, 83(9): 1393-411.e7.
[11] ZHANG J, CHEN W, MA W, HAN C, SONG K, KWON H, et al. EZH2 Promotes Cholangiocarcinoma Development and Progression through Histone Methylation and microRNA-Mediated Down-Regulation of Tumor Suppressor Genes [J]. Am J Pathol, 2022, 192(12): 1712-24.
[12] WANG K, JIANG X, JIANG Y, LIU J, DU Y, ZHANG Z, et al. EZH2-H3K27me3-mediated silencing of mir-139-5p inhibits cellular senescence in hepatocellular carcinoma by activating TOP2A [J]. J Exp Clin Cancer Res, 2023, 42(1): 320.
[13] KIM E, KIM M, WOO D H, SHIN Y, SHIN J, CHANG N, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells [J]. Cancer Cell, 2013, 23(6): 839-52.
[14] VASANTHAKUMAR A, XU D, LUN A T, KUEH A J, VAN GISBERGEN K P, IANNARELLA N, et al. A non-canonical function of Ezh2 preserves immune homeostasis [J]. EMBO Rep, 2017, 18(4): 619-31.
[15] LIU Q, WANG G, LI Q, JIANG W, KIM J S, WANG R, et al. Polycomb group proteins EZH2 and EED directly regulate androgen receptor in advanced prostate cancer [J]. Int J Cancer, 2019, 145(2): 415-26.
[16] YANG Z, WEI B, QIAO A, YANG P, CHEN W, ZHEN D, et al. A novel EZH2/NXPH4/CDKN2A axis is involved in regulating the proliferation and migration of non-small cell lung cancer cells [J]. Biosci Biotechnol Biochem, 2022, 86(3): 340-50.
[17] CHEN J, HONG J H, HUANG Y, LIU S, YIN J, DENG P, et al. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer [J]. Mol Cancer, 2023, 22(1): 85.
[18] PAWLYN C, BRIGHT M D, BUROS A F, STEIN C K, WALTERS Z, ARONSON L I, et al. Overexpression of EZH2 in multiple myeloma is associated with poor prognosis and dysregulation of cell cycle control [J]. Blood Cancer J, 2017, 7(3): e549.
[19] XU X, GU J, DING X, GE G, ZANG X, JI R, et al. LINC00978 promotes the progression of hepatocellular carcinoma by regulating EZH2-mediated silencing of p21 and E-cadherin expression [J]. Cell Death Dis, 2019, 10(10): 752.
[20] CHEN W M, CHEN W D, JIANG X M, JIA X F, WANG H M, ZHANG Q J, et al. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer [J]. World J Gastroenterol, 2017, 23(33): 6100-10.
[21] ZOU G, HUANG Y, ZHANG S, KO K P, KIM B, ZHANG J, et al. E-cadherin loss drives diffuse-type gastric tumorigenesis via EZH2-mediated reprogramming [J]. J Exp Med, 2024, 221(4).
[22] ZHUANG C, WANG P, HUANG D, XU L, WANG X, WANG L, et al. A double-negative feedback loop between EZH2 and miR-26a regulates tumor cell growth in hepatocellular carcinoma [J]. Int J Oncol, 2016, 48(3): 1195-204.
[23] BAI B, LIU Y, FU X M, QIN H Y, LI G K, WANG H C, et al. Dysregulation of EZH2/miR-138-5p Axis Contributes to Radiosensitivity in Hepatocellular Carcinoma Cell by Downregulating Hypoxia-Inducible Factor 1 Alpha (HIF-1α) [J]. Oxid Med Cell Longev, 2022, 2022: 7608712.
[24] ZHAO J, LI H, ZHAO S, WANG E, ZHU J, FENG D, et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling [J]. Mol Cancer, 2021, 20(1): 46.
[25] DENG M, WANG Z, LUO J, CAO H, LI Y, CHEN L, et al. CircZNF367 promotes osteoclast differentiation and osteoporosis by interacting with FUS to maintain CRY2 mRNA stability [J]. J Orthop Surg Res, 2023, 18(1): 492.
[26] WANG S, CHEN J, LI P, CHEN Y. LINC01133 can induce acquired ferroptosis resistance by enhancing the FSP1 mRNA stability through forming the LINC01133-FUS-FSP1 complex [J]. Cell Death Dis, 2023, 14(11): 767.
[27] XING C Y, ZHANG Y Z, HU W, ZHAO L Y. LINC00313 facilitates osteosarcoma carcinogenesis and metastasis through enhancing EZH2 mRNA stability and EZH2-mediated silence of PTEN expression [J]. Cell Mol Life Sci, 2022, 79(7): 382.
[28] WU T, WANG G, ZENG X, SUN Z, LI S, WANG W, et al. Hsa_circ_0006232 promotes laryngeal squamous cell cancer progression through FUS-mediated EZH2 stabilization [J]. Cell Cycle, 2021, 20(18): 1799-811.
[29] DI W, WEINAN X, XIN L, ZHIWEI Y, XINYUE G, JINXUE T, et al. Long noncoding RNA SNHG14 facilitates colorectal cancer metastasis through targeting EZH2-regulated EPHA7 [J]. Cell Death Dis, 2019, 10(7): 514.
[30] SOUROULLAS G P, JECK W R, PARKER J S, SIMON J M, LIU J Y, PAULK J, et al. An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation [J]. Nat Med, 2016, 22(6): 632-40.
[31] SNEERINGER C J, SCOTT M P, KUNTZ K W, KNUTSON S K, POLLOCK R M, RICHON V M, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas [J]. Proc Natl Acad Sci U S A, 2010, 107(49): 20980-5.
[32] SCORE J, HIDALGO-CURTIS C, JONES A V, WINKELMANN N, SKINNER A, WARD D, et al. Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms [J]. Blood, 2012, 119(5): 1208-13.
[33] ZHANG D, QIU Y, ZHANG W, DU D, LIU Y, LIU L, et al. Homeobox B9 promotes the invasion and metastasis of hepatocellular carcinoma cells via the EZH2-MIR203A-SNAI2 axis [J]. J Transl Med, 2024, 22(1): 918.
[34] ZHENG M, JIANG Y P, CHEN W, LI K D, LIU X, GAO S Y, et al. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma [J]. Oncotarget, 2015, 6(9): 6797-810.
[35] LU L, LUO F, LIU Y, LIU X, SHI L, LU X, et al. Posttranscriptional silencing of the lncRNA MALAT1 by miR-217 inhibits the epithelial-mesenchymal transition via enhancer of zeste homolog 2 in the malignant transformation of HBE cells induced by cigarette smoke extract [J]. Toxicol Appl Pharmacol, 2015, 289(2): 276-85.
[36] ZHANG K, FANG T, SHAO Y, WU Y. TGF-β-MTA1-SMAD7-SMAD3-SOX4-EZH2 Signaling Axis Promotes Viability, Migration, Invasion and EMT of Hepatocellular Carcinoma Cells [J]. Cancer Manag Res, 2021, 13: 7087-99.
[37] ZANG C, NIE F Q, WANG Q, SUN M, LI W, HE J, et al. Long non-coding RNA LINC01133 represses KLF2, P21 and E-cadherin transcription through binding with EZH2, LSD1 in non small cell lung cancer [J]. Oncotarget, 2016, 7(10): 11696-707.
[38] ZHANG S, LIAO W, WU Q, HUANG X, PAN Z, CHEN W, et al. LINC00152 upregulates ZEB1 expression and enhances epithelial-mesenchymal transition and oxaliplatin resistance in esophageal cancer by interacting with EZH2 [J]. Cancer Cell Int, 2020, 20(1): 569.
[39] LIN Y H, WU M H, LIU Y C, LYU P C, YEH C T, LIN K H. LINC01348 suppresses hepatocellular carcinoma metastasis through inhibition of SF3B3-mediated EZH2 pre-mRNA splicing [J]. Oncogene, 2021, 40(28): 4675-85.
[40] VERMA A, SINGH A, SINGH M P, NENGROO M A, SAINI K K, SATRUSAL S R, et al. EZH2-H3K27me3 mediated KRT14 upregulation promotes TNBC peritoneal metastasis [J]. Nat Commun, 2022, 13(1): 7344.
[41] CARLSON S M, GOZANI O. Emerging technologies to map the protein methylome [J]. J Mol Biol, 2014, 426(20): 3350-62.
[42] LIU F, MA F, WANG Y, HAO L, ZENG H, JIA C, et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis [J]. Nat Cell Biol, 2017, 19(11): 1358-70.
[43] PAN T, HAO J, WANG Y, DUAN W, YUE L, GAO X. Role in post-translational modification of M2-type pyruvate kinase in tumorigenesis and development [J]. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2023, 48(9): 1359-67.
[44] HUANG H, OUYANG Q, MEI K, LIU T, SUN Q, LIU W, et al. Acetylation of SCFD1 regulates SNARE complex formation and autophagosome-lysosome fusion [J]. Autophagy, 2023, 19(1): 189-203.
[45] YI F, CAI C, RUAN B, HAO M, YEO S K, HAAS M, et al. Regulation of RB1CC1/FIP200 stability and autophagy function by CREBBP-mediated acetylation in an intrinsically disordered region [J]. Autophagy, 2023, 19(6): 1662-77.
[46] HUANG C, HU F, SONG D, SUN X, LIU A, WU Q, et al. EZH2-triggered methylation of SMAD3 promotes its activation and tumor metastasis [J]. J Clin Invest, 2022, 132(5).
[47] HU X, LIU Y, SHEN H, ZHANG T, LIANG T. MTF2 facilitates the advancement of osteosarcoma through mediating EZH2/SFRP1/Wnt signaling [J]. J Orthop Surg Res, 2024, 19(1): 467.
[48] CHEN W, WANG H T, JI J F, WANG Z Y, SHI T, WU M H, et al. Epigenetic network of EZH2/SFRP1/Wnt in the epithelial-mesenchymal transition of laryngeal carcinoma cells [J]. Neoplasma, 2022, 69(3): 680-90.
[49] ZHANG Y, LIN C, LIAO G, LIU S, DING J, TANG F, et al. MicroRNA-506 suppresses tumor proliferation and metastasis in colon cancer by directly targeting the oncogene EZH2 [J]. Oncotarget, 2015, 6(32): 32586-601.
[50] LIANG G, HAN L, QU M, XUE J, XU D, WU X, et al. Down-regulation of EZH2 genes targeting RUNX3 affects proliferation, invasion, and metastasis of human colon cancer cells by Wnt/β-catenin signaling pathway [J]. Aging (Albany NY), 2023, 15(23): 13655-68.
[51] REN L, DENG H, JIANG Y, LIU C. Dual-Regulated Mechanism of EZH2 and KDM6A on SALL4 Modulates Tumor Progression via Wnt/β-Catenin Pathway in Gastric Cancer [J]. Dig Dis Sci, 2023, 68(4): 1292-305.
[53] ZINGG D, DEBBACHE J, PEñA-HERNáNDEZ R, ANTUNES A T, SCHAEFER S M, CHENG P F, et al. EZH2-Mediated Primary Cilium Deconstruction Drives Metastatic Melanoma Formation [J]. Cancer Cell, 2018, 34(1): 69-84.e14.
[54] XU H, ZHAO G, ZHANG Y, JIANG H, WANG W, ZHAO D, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2 [J]. Stem Cell Res Ther, 2019, 10(1): 381.
[55] LIAN R, MA H, WU Z, ZHANG G, JIAO L, MIAO W, et al. EZH2 promotes cell proliferation by regulating the expression of RUNX3 in laryngeal carcinoma [J]. Mol Cell Biochem, 2018, 439(1-2): 35-43.
[56] CHEN Q, ZHENG P S, YANG W T. EZH2-mediated repression of GSK-3β and TP53 promotes Wnt/β-catenin signaling-dependent cell expansion in cervical carcinoma [J]. Oncotarget, 2016, 7(24): 36115-29.
[57] ZHANG J J, CHEN J T, HUA L, YAO K H, WANG C Y. miR-98 inhibits hepatocellular carcinoma cell proliferation via targeting EZH2 and suppressing Wnt/β-catenin signaling pathway [J]. Biomed Pharmacother, 2017, 85: 472-8.
[58] WANG Y F, YU L, HU Z L, FANG Y F, SHEN Y Y, SONG M F, et al. Regulation of CCL2 by EZH2 affects tumor-associated macrophages polarization and infiltration in breast cancer [J]. Cell Death Dis, 2022, 13(8): 748.
[59] ISSHIKI Y, CHEN X, TEATER M, KARAGIANNIDIS I, NAM H, CAI W, et al. EZH2 inhibition enhances T cell immunotherapies by inducing lymphoma immunogenicity and improving T cell function [J]. Cancer Cell, 2025, 43(1): 49-68.e9.
[60] PORAZZI P, NASON S, YANG Z, CARTURAN A, GHILARDI G, GURUPRASAD P, et al. EZH1/EZH2 inhibition enhances adoptive T cell immunotherapy against multiple cancer models [J]. Cancer Cell, 2025, 43(3): 537-51.e7.
[61] XIE Y, LIN H, WEI W, KONG Y, FANG Q, CHEN E, et al. LINC00839 promotes malignancy of liver cancer via binding FMNL2 under hypoxia [J]. Sci Rep, 2022, 12(1): 18757.
[62] HUANG Z, TANG Y, ZHANG J, HUANG J, CHENG R, GUO Y, et al. Hypoxia makes EZH2 inhibitor not easy-advances of crosstalk between HIF and EZH2 [J]. Life Metab, 2024, 3(4).
[63] HWANG-VERSLUES W W, CHANG P H, JENG Y M, KUO W H, CHIANG P H, CHANG Y C, et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy [J]. Proc Natl Acad Sci U S A, 2013, 110(30): 12331-6.
[64] SHAN L, ZHOU X, LIU X, WANG Y, SU D, HOU Y, et al. FOXK2 Elicits Massive Transcription Repression and Suppresses the Hypoxic Response and Breast Cancer Carcinogenesis [J]. Cancer Cell, 2016, 30(5): 708-22.
[65] SUN J, CAI X, YUNG M M, ZHOU W, LI J, ZHANG Y, et al. miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer [J]. Oncogene, 2019, 38(4): 564-80.
[66] XU S, LI X, LI L, WANG Y, GENG C, GUO F, et al. CTCF-silenced miR-137 contributes to EMT and radioresistance in esophageal squamous cell carcinoma [J]. Cancer Cell Int, 2021, 21(1): 155.
[67] LIU H, LI W, YU X, GAO F, DUAN Z, MA X, et al. EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis [J]. Oncotarget, 2016, 7(35): 56338-54.
[68] ZHONG J, YANG X, CHEN J, HE K, GAO X, WU X, et al. Circular EZH2-encoded EZH2-92aa mediates immune evasion in glioblastoma via inhibition of surface NKG2D ligands [J]. Nat Commun, 2022, 13(1): 4795.
[69] LIU J, YANG L, LIU X, LIU L, LIU M, FENG X, et al. lncRNA HOTTIP Recruits EZH2 to Inhibit PTEN Expression and Participates in IM Resistance in Chronic Myeloid Leukemia [J]. Stem Cells Int, 2022, 2022: 9993393.
[70] HAYWARD N K, WILMOTT J S, WADDELL N, JOHANSSON P A, FIELD M A, NONES K, et al. Whole-genome landscapes of major melanoma subtypes [J]. Nature, 2017, 545(7653): 175-80.
[71] HODIS E, WATSON I R, KRYUKOV G V, AROLD S T, IMIELINSKI M, THEURILLAT J P, et al. A landscape of driver mutations in melanoma [J]. Cell, 2012, 150(2): 251-63.
[72] SINI M C, DONEDDU V, PALIOGIANNIS P, CASULA M, COLOMBINO M, MANCA A, et al. Genetic alterations in main candidate genes during melanoma progression [J]. Oncotarget, 2018, 9(9): 8531-41.
[73] TANDA E T, VANNI I, BOUTROS A, ANDREOTTI V, BRUNO W, GHIORZO P, et al. Current State of Target Treatment in BRAF Mutated Melanoma [J]. Front Mol Biosci, 2020, 7: 154.
[74] ZHANG Y, DU X L, WANG C J, LIN D C, RUAN X, FENG Y B, et al. Reciprocal activation between PLK1 and Stat3 contributes to survival and proliferation of esophageal cancer cells [J]. Gastroenterology, 2012, 142(3): 521-30.e3.
[75] UEBEL A, KEWITZ-HEMPEL S, WILLSCHER E, GEBHARDT K, SUNDERKöTTER C, GERLOFF D. Resistance to BRAF Inhibitors: EZH2 and Its Downstream Targets as Potential Therapeutic Options in Melanoma [J]. Int J Mol Sci, 2023, 24(3).
[76] YANG C, GU Y, GE Z, SONG C. Targeting EZH2 Promotes Chemosensitivity of BCL-2 Inhibitor through Suppressing PI3K and c-KIT Signaling in Acute Myeloid Leukemia [J]. Int J Mol Sci, 2022, 23(19).
[77] WANG X, XIE Z, LOU Z, CHEN Y, HUANG S, REN Y, et al. Regulation of the PTEN/PI3K/AKT pathway in RCC using the active compounds of natural products in vitro [J]. Mol Med Rep, 2021, 24(5).
[78] SU Y, WANG B, HUANG J, HUANG M, LIN T. YTHDC1 positively regulates PTEN expression and plays a critical role in cisplatin resistance of bladder cancer [J]. Cell Prolif, 2023, 56(7): e13404.
[79] DENG X, KONG F, LI S, JIANG H, DONG L, XU X, et al. A KLF4/PiHL/EZH2/HMGA2 regulatory axis and its function in promoting oxaliplatin-resistance of colorectal cancer [J]. Cell Death Dis, 2021, 12(5): 485.
[80] VESCHI V, VERONA F, THIELE C J. Cancer Stem Cells and Neuroblastoma: Characteristics and Therapeutic Targeting Options [J]. Front Endocrinol (Lausanne), 2019, 10: 782.
[81] KAMIJO T. Role of stemness-related molecules in neuroblastoma [J]. Pediatr Res, 2012, 71(4 Pt 2): 511-5.
[82] ADHIKARY G, GRUN D, BALASUBRAMANIAN S, KERR C, HUANG J M, ECKERT R L. Survival of skin cancer stem cells requires the Ezh2 polycomb group protein [J]. Carcinogenesis, 2015, 36(7): 800-10.
[83] EL BABA R, PASQUEREAU S, HAIDAR AHMAD S, DIAB-ASSAF M, HERBEIN G. Oncogenic and Stemness Signatures of the High-Risk HCMV Strains in Breast Cancer Progression [J]. Cancers (Basel), 2022, 14(17).
[84] LIU H, SUN Q, SUN Y, ZHANG J, YUAN H, PANG S, et al. MELK and EZH2 Cooperate to Regulate Medulloblastoma Cancer Stem-like Cell Proliferation and Differentiation [J]. Mol Cancer Res, 2017, 15(9): 1275-86.
[85] CHEN L, ZHANG M, FANG L, YANG X, CAO N, XU L, et al. Coordinated regulation of the ribosome and proteasome by PRMT1 in the maintenance of neural stemness in cancer cells and neural stem cells [J]. J Biol Chem, 2021, 297(5): 101275.
[86] VAN VLERKEN L E, KIEFER C M, MOREHOUSE C, LI Y, GROVES C, WILSON S D, et al. EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter [J]. Stem Cells Transl Med, 2013, 2(1): 43-52.
[87] SUVà M L, RIGGI N, JANISZEWSKA M, RADOVANOVIC I, PROVERO P, STEHLE J C, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance [J]. Cancer Res, 2009, 69(24): 9211-8.
[88] ZONG X, NEPHEW K P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting [J]. Cancers (Basel), 2019, 11(7).
[89] GORODETSKA I, LUKIYANCHUK V, PEITZSCH C, KOZERETSKA I, DUBROVSKA A. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype [J]. Int J Cancer, 2019, 145(11): 2974-85.
[90] CHEN Z, TANG W, ZHOU Y, HE Z. The role of LINC01419 in regulating the cell stemness in lung adenocarcinoma through recruiting EZH2 and regulating FBP1 expression [J]. Biol Direct, 2022, 17(1): 23.
[91] TSENG C F, CHEN L T, WANG H D, LIU Y H, SHIAH S G. Transcriptional suppression of Dicer by HOXB-AS3/EZH2 complex dictates sorafenib resistance and cancer stemness [J]. Cancer Sci, 2022, 113(5): 1601-12.
[92] ZHANG Y, TANG B, SONG J, YU S, LI Y, SU H, et al. Lnc-PDZD7 contributes to stemness properties and chemosensitivity in hepatocellular carcinoma through EZH2-mediated ATOH8 transcriptional repression [J]. J Exp Clin Cancer Res, 2019, 38(1): 92.
[93] LIN H, XIE Y, KONG Y, YANG L, LI M. Identification of Two Molecular Subtypes of Hepatocellular Carcinoma Based on Dysregulated Immune LncRNAs [J]. Front Mol Biosci, 2021, 8: 625858.
[94] CAI L, LIU Y, LI Y, LIU B, CAO Y, YANG W, et al. TRIM37 interacts with EZH2 to epigenetically suppress PTCH1 and regulate stemness in glioma stem cells through sonic hedgehog pathway [J]. J Neurooncol, 2024, 169(2): 269-79.
[95] MARIE S K, SHINJO S M. Metabolism and brain cancer [J]. Clinics (Sao Paulo), 2011, 66 Suppl 1(Suppl 1): 33-43.
[96] LIU Q, WANG L, WANG Z, YANG Y, TIAN J, LIU G, et al. GRIM-19 opposes reprogramming of glioblastoma cell metabolism via HIF1α destabilization [J]. Carcinogenesis, 2013, 34(8): 1728-36.
[97] ZHANG T, GONG Y, MENG H, LI C, XUE L. Symphony of epigenetic and metabolic regulation-interaction between the histone methyltransferase EZH2 and metabolism of tumor [J]. Clin Epigenetics, 2020, 12(1): 72.
[98] PASCAL L E, AI J, RIGATTI L H, LIPTON A K, XIAO W, GNARRA J R, et al. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate [J]. Angiogenesis, 2011, 14(3): 331-43.
[99] CHEN F, CHEN J, YANG L, LIU J, ZHANG X, ZHANG Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells [J]. Nat Cell Biol, 2019, 21(4): 498-510.
[100] PANG B, ZHENG X R, TIAN J X, GAO T H, GU G Y, ZHANG R, et al. EZH2 promotes metabolic reprogramming in glioblastomas through epigenetic repression of EAF2-HIF1α signaling [J]. Oncotarget, 2016, 7(29): 45134-43.
[101] WANG Y, WANG M, WEI W, HAN D, CHEN X, HU Q, et al. Disruption of the EZH2/miRNA/β-catenin signaling suppresses aerobic glycolysis in glioma [J]. Oncotarget, 2016, 7(31): 49450-8.
[102] ZHOU J, LIN Y, KANG X, LIU Z, ZOU J, XU F. Hypoxia-mediated promotion of glucose metabolism in non-small cell lung cancer correlates with activation of the EZH2/FBXL7/PFKFB4 axis [J]. Cell Death Dis, 2023, 14(5): 326.
[103] WANG J, YANG C, XU H, FAN X, JIA L, DU Y, et al. The Interplay Between HIF-1α and EZH2 in Lung Cancer and Dual-Targeted Drug Therapy [J]. Adv Sci (Weinh), 2024, 11(7): e2303904.
[104] VANTAKU V, PUTLURI V, BADER D A, MAITY S, MA J, ARNOLD J M, et al. Epigenetic loss of AOX1 expression via EZH2 leads to metabolic deregulations and promotes bladder cancer progression [J]. Oncogene, 2020, 39(40): 6265-85.
[105] GAN L, LI Q, NIE W, ZHANG Y, JIANG H, TAN C, et al. PROX1-mediated epigenetic silencing of SIRT3 contributes to proliferation and glucose metabolism in colorectal cancer [J]. Int J Biol Sci, 2023, 19(1): 50-65.
[106] CURRIE E, SCHULZE A, ZECHNER R, WALTHER T C, FARESE R V, JR. Cellular fatty acid metabolism and cancer [J]. Cell Metab, 2013, 18(2): 153-61.
[107] AHMAD F, PATRICK S, SHEIKH T, SHARMA V, PATHAK P, MALGULWAR P B, et al. Telomerase reverse transcriptase (TERT) - enhancer of zeste homolog 2 (EZH2) network regulates lipid metabolism and DNA damage responses in glioblastoma [J]. J Neurochem, 2017, 143(6): 671-83.
[108] YIEW N K H, GREENWAY C, ZARZOUR A, AHMADIEH S, GOO B, KIM D, et al. Enhancer of zeste homolog 2 (EZH2) regulates adipocyte lipid metabolism independent of adipogenic differentiation: Role of apolipoprotein E [J]. J Biol Chem, 2019, 294(21): 8577-91.
[109] HAYDEN A, JOHNSON P W, PACKHAM G, CRABB S J. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin A analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition [J]. Breast Cancer Res Treat, 2011, 127(1): 109-19.
[110] YANG Y, YANG T, ZHAO Z, ZHANG H, YUAN P, WANG G, et al. Down-regulation of BMAL1 by MiR-494-3p Promotes Hepatocellular Carcinoma Growth and Metastasis by Increasing GPAM-mediated Lipid Biosynthesis [J]. Int J Biol Sci, 2022, 18(16): 6129-44.
[111] ZHANG Y, WU M J, LU W C, LI Y C, CHANG C J, YANG J Y. Metabolic switch regulates lineage plasticity and induces synthetic lethality in triple-negative breast cancer [J]. Cell Metab, 2024, 36(1): 193-208.e8.
[112] BAKHTIARI M, JORDAN S C, MUMME H L, SHARMA R, SHANMUGAM M, BHASIN S S, et al. ARMH1 is a novel marker associated with poor pediatric AML outcomes that affect the fatty acid synthesis and cell cycle pathways [J]. Front Oncol, 2024, 14: 1445173.
[113] SAHU U, MULLARKEY M P, MURPHY S A, ANDERSON J C, PUTLURI V, KAMAL A H M, et al. IDH status dictates oHSV mediated metabolic reprogramming affecting anti-tumor immunity [J]. Nat Commun, 2025, 16(1): 3874.
[114] ZHANG T, GUO Z, HUO X, GONG Y, LI C, HUANG J, et al. Dysregulated lipid metabolism blunts the sensitivity of cancer cells to EZH2 inhibitor [J]. EBioMedicine, 2022, 77: 103872.
[115] NOCITO M C, HANTEL C, LERARIO A M, MASTROROCCO F, DE MARTINO L, MUSICCO C, et al. A targetable antioxidant defense mechanism to EZH2 inhibitors enhances tumor cell vulnerability to ferroptosis [J]. Cell Death Dis, 2025, 16(1): 291.
[116] LIU Y, TU C E, GUO X, WU C, GU C, LAI Q, et al. Tumor-suppressive function of EZH2 is through inhibiting glutaminase [J]. Cell Death Dis, 2021, 12(11): 975.
[117] PAPATHANASSIU A E, KO J H, IMPRIALOU M, BAGNATI M, SRIVASTAVA P K, VU H A, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases [J]. Nat Commun, 2017, 8: 16040.
[119] T W M F, ISLAM J M M, HIGASHI R M, LIN P, BRAINSON C F, LANE A N. Metabolic reprogramming driven by EZH2 inhibition depends on cell-matrix interactions [J]. J Biol Chem, 2024, 300(1): 105485.
[120] CANTLEY L C. The phosphoinositide 3-kinase pathway [J]. Science, 2002, 296(5573): 1655-7.
[121] WANG X Q, SUN P, PALLER A S. Inhibition of integrin-linked kinase/protein kinase B/Akt signaling: mechanism for ganglioside-induced apoptosis [J]. J Biol Chem, 2001, 276(48): 44504-11.
[122] LI Y, SONG Y H, MOHLER J, DELAFONTAINE P. ANG II induces apoptosis of human vascular smooth muscle via extrinsic pathway involving inhibition of Akt phosphorylation and increased FasL expression [J]. Am J Physiol Heart Circ Physiol, 2006, 290(5): H2116-23.
[123] TIAN Y, CHEN Z H, WU P, ZHANG D, MA Y, LIU X F, et al. MIR497HG-Derived miR-195 and miR-497 Mediate Tamoxifen Resistance via PI3K/AKT Signaling in Breast Cancer [J]. Adv Sci (Weinh), 2023, 10(12): e2204819.
[124] LI H, SHEN X, MA M, LIU W, YANG W, WANG P, et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway [J]. J Exp Clin Cancer Res, 2021, 40(1): 340.
[125] KONG C, WU M, LU Q, KE B, XIE J, LI A. PI3K/AKT confers intrinsic and acquired resistance to pirtobrutinib in chronic lymphocytic leukemia [J]. Leuk Res, 2024, 144: 107548.
[126] SANCHES J G P, SONG B, ZHANG Q, CUI X, YABASIN I B, NTIM M, et al. The Role of KDM2B and EZH2 in Regulating the Stemness in Colorectal Cancer Through the PI3K/AKT Pathway [J]. Front Oncol, 2021, 11: 637298.
[127] DAI Q, ZHANG T, PAN J, LI C. LncRNA UCA1 promotes cisplatin resistance in gastric cancer via recruiting EZH2 and activating PI3K/AKT pathway [J]. J Cancer, 2020, 11(13): 3882-92.
[128] CHEN J, WANG F, XU H, XU L, CHEN D, WANG J, et al. Long Non-Coding RNA SNHG1 Regulates the Wnt/β-Catenin and PI3K/AKT/mTOR Signaling Pathways via EZH2 to Affect the Proliferation, Apoptosis, and Autophagy of Prostate Cancer Cell [J]. Front Oncol, 2020, 10: 552907.
[129] XU K, WU Z J, GRONER A C, HE H H, CAI C, LIS R T, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent [J]. Science, 2012, 338(6113): 1465-9.
[130] WEI X, GUO J, LI Q, JIA Q, JING Q, LI Y, et al. Bach1 regulates self-renewal and impedes mesendodermal differentiation of human embryonic stem cells [J]. Sci Adv, 2019, 5(3): eaau7887.
[131] BASU S, CHERIYAMUNDATH S, BEN-ZE'EV A. Cell-cell adhesion: linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis [J]. F1000Res, 2018, 7.
[132] CAO W, LEE H, WU W, ZAMAN A, MCCORKLE S, YAN M, et al. Multi-faceted epigenetic dysregulation of gene expression promotes esophageal squamous cell carcinoma [J]. Nat Commun, 2020, 11(1): 3675.
[133] FAZI B, GARBO S, TOSCHI N, MANGIOLA A, LOMBARI M, SICARI D, et al. The lncRNA H19 positively affects the tumorigenic properties of glioblastoma cells and contributes to NKD1 repression through the recruitment of EZH2 on its promoter [J]. Oncotarget, 2018, 9(21): 15512-25.
[134] KHAN H, NI Z, FENG H, XING Y, WU X, HUANG D, et al. Combination of curcumin with N-n-butyl haloperidol iodide inhibits hepatocellular carcinoma malignant proliferation by downregulating enhancer of zeste homolog 2 (EZH2) - lncRNA H19 to silence Wnt/β-catenin signaling [J]. Phytomedicine, 2021, 91: 153706.
[135] YU T, ZHOU F, TIAN W, XU R, WANG B, ZENG A, et al. EZH2 interacts with HP1BP3 to epigenetically activate WNT7B that promotes temozolomide resistance in glioblastoma [J]. Oncogene, 2023, 42(6): 461-70.
[136] JUNG H Y, JUN S, LEE M, KIM H C, WANG X, JI H, et al. PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation [J]. Mol Cell, 2013, 52(2): 193-205.
[137] YU D, WANG S, WANG J, ZHANG K, NIU Z, LIN N. EZH2-STAT3 signaling pathway regulates GSDMD-mediated pyroptosis in glioblastoma [J]. Cell Death Discov, 2024, 10(1): 341.
[138] ZHANG D, YANG X J, LUO Q D, FU D L, LI H L, LI H C, et al. EZH2 enhances the invasive capability of renal cell carcinoma cells via activation of STAT3 [J]. Mol Med Rep, 2018, 17(3): 3621-6.
[139] LI L, ZHANG Y, YANG K, LIU W, ZHOU Z, XU Y. miRNA-449c-5p regulates the JAK-STAT pathway in inhibiting cell proliferation and invasion in human breast cancer cells by targeting ERBB2 [J]. Cancer Rep (Hoboken), 2024, 7(2): e1974.
[140] SAKAHARA M, OKAMOTO T, OYANAGI J, TAKANO H, NATSUME Y, YAMANAKA H, et al. IFN/STAT signaling controls tumorigenesis and the drug response in colorectal cancer [J]. Cancer Sci, 2019, 110(4): 1293-305.
[141] ZHENG M, CAO M X, LUO X J, LI L, WANG K, WANG S S, et al. EZH2 promotes invasion and tumour glycolysis by regulating STAT3 and FoxO1 signalling in human OSCC cells [J]. J Cell Mol Med, 2019, 23(10): 6942-54.
[142] GU C J, XIE F, ZHANG B, YANG H L, CHENG J, HE Y Y, et al. High Glucose Promotes Epithelial-Mesenchymal Transition of Uterus Endometrial Cancer Cells by Increasing ER/GLUT4-Mediated VEGF Secretion [J]. Cell Physiol Biochem, 2018, 50(2): 706-20.
[143] XU Q, ZHANG Q, ISHIDA Y, HAJJAR S, TANG X, SHI H, et al. EGF induces epithelial-mesenchymal transition and cancer stem-like cell properties in human oral cancer cells via promoting Warburg effect [J]. Oncotarget, 2017, 8(6): 9557-71.
[144] LI C, SONG J, GUO Z, GONG Y, ZHANG T, HUANG J, et al. EZH2 Inhibitors Suppress Colorectal Cancer by Regulating Macrophage Polarization in the Tumor Microenvironment [J]. Front Immunol, 2022, 13: 857808.
[145] ZHOU X, CHEN H, HU Y, MA X, LI J, SHI Y, et al. Enhancer of zeste homolog 2 promotes renal fibrosis after acute kidney injury by inducing epithelial-mesenchymal transition and activation of M2 macrophage polarization [J]. Cell Death Dis, 2023, 14(4): 253.
[146] YANG Q, ZHAO S, SHI Z, CAO L, LIU J, PAN T, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling [J]. J Exp Clin Cancer Res, 2021, 40(1): 120.
[147] FUJII S, TOKITA K, WADA N, ITO K, YAMAUCHI C, ITO Y, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes [J]. Oncogene, 2011, 30(39): 4118-28.
[148] CAI Y, CHEN P, XU H, LI S, ZHAO B, FAN Y, et al. EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway [J]. Int J Mol Sci, 2023, 24(13).
[149] ZHANG Q, SHI Y, LIU S, YANG W, CHEN H, GUO N, et al. EZH2/G9a interact to mediate drug resistance in non-small-cell lung cancer by regulating the SMAD4/ERK/c-Myc signaling axis [J]. Cell Rep, 2024, 43(2): 113714.
[150] HOXHA S, SHEPARD A, TROUTMAN S, DIAO H, DOHERTY J R, JANISZEWSKA M, et al. YAP-Mediated Recruitment of YY1 and EZH2 Represses Transcription of Key Cell-Cycle Regulators [J]. Cancer Res, 2020, 80(12): 2512-22.
[151] LO SARDO F, TURCO C, MESSINA B, SACCONI A, AUCIELLO F R, PULITO C, et al. The oncogenic axis YAP/MYC/EZH2 impairs PTEN tumor suppression activity enhancing lung tumorigenicity [J]. Cell Death Discov, 2024, 10(1): 452.
[152] LUO C, BALSA E, PERRY E A, LIANG J, TAVARES C D, VAZQUEZ F, et al. H3K27me3-mediated PGC1α gene silencing promotes melanoma invasion through WNT5A and YAP [J]. J Clin Invest, 2020, 130(2): 853-62.
[153] JEONG G Y, PARK M K, CHOI H J, AN H W, PARK Y U, CHOI H J, et al. NSD3-Induced Methylation of H3K36 Activates NOTCH Signaling to Drive Breast Tumor Initiation and Metastatic Progression [J]. Cancer Res, 2021, 81(1): 77-90.
[154] QIAN X, ZHANG Y. EZH2 enhances proliferation and migration of trophoblast cell lines by blocking GADD45A-mediated p38/MAPK signaling pathway [J]. Bioengineered, 2022, 13(5): 12583-97.
[1] WU Z, XIA F, LIN R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980-2021: a systematic analysis for the GBD 2021 [J]. J Hematol Oncol, 2024, 17(1): 119.
[2] KENTSIS A. Why do young people get cancer? [J]. Pediatr Blood Cancer, 2020, 67(7): e28335.
[3] ELANGOVAN A, LI Y, PIRES D E V, DAVIS M J, VERSPOOR K. Large-scale protein-protein post-translational modification extraction with distant supervision and confidence calibrated BioBERT [J]. BMC Bioinformatics, 2022, 23(1): 4.
[4] SEPICH-POORE G D, ZITVOGEL L, STRAUSSMAN R, HASTY J, WARGO J A, KNIGHT R. The microbiome and human cancer [J]. Science, 2021, 371(6536).
[5] NISHIYAMA A, NAKANISHI M. Navigating the DNA methylation landscape of cancer [J]. Trends Genet, 2021, 37(11): 1012-27.
[6] CAO J, YAN Q. Cancer Epigenetics, Tumor Immunity, and Immunotherapy [J]. Trends Cancer, 2020, 6(7): 580-92.
[7] MENON A, ABD-AZIZ N, KHALID K, POH C L, NAIDU R. miRNA: A Promising Therapeutic Target in Cancer [J]. Int J Mol Sci, 2022, 23(19).
[8] TANG J, ZHUANG S. Histone acetylation and DNA methylation in ischemia/reperfusion injury [J]. Clin Sci (Lond), 2019, 133(4): 597-609.
[9] JIANG L, HUANG L, JIANG W. H3K27me3-mediated epigenetic regulation in pluripotency maintenance and lineage differentiation [J]. Cell Insight, 2024, 3(4): 100180.
[10] GLANCY E, WANG C, TUCK E, HEALY E, AMATO S, NEIKES H K, et al. PRC2.1- and PRC2.2-specific accessory proteins drive recruitment of different forms of canonical PRC1 [J]. Mol Cell, 2023, 83(9): 1393-411.e7.
[11] ZHANG J, CHEN W, MA W, HAN C, SONG K, KWON H, et al. EZH2 Promotes Cholangiocarcinoma Development and Progression through Histone Methylation and microRNA-Mediated Down-Regulation of Tumor Suppressor Genes [J]. Am J Pathol, 2022, 192(12): 1712-24.
[12] WANG K, JIANG X, JIANG Y, LIU J, DU Y, ZHANG Z, et al. EZH2-H3K27me3-mediated silencing of mir-139-5p inhibits cellular senescence in hepatocellular carcinoma by activating TOP2A [J]. J Exp Clin Cancer Res, 2023, 42(1): 320.
[13] KIM E, KIM M, WOO D H, SHIN Y, SHIN J, CHANG N, et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells [J]. Cancer Cell, 2013, 23(6): 839-52.
[14] VASANTHAKUMAR A, XU D, LUN A T, KUEH A J, VAN GISBERGEN K P, IANNARELLA N, et al. A non-canonical function of Ezh2 preserves immune homeostasis [J]. EMBO Rep, 2017, 18(4): 619-31.
[15] LIU Q, WANG G, LI Q, JIANG W, KIM J S, WANG R, et al. Polycomb group proteins EZH2 and EED directly regulate androgen receptor in advanced prostate cancer [J]. Int J Cancer, 2019, 145(2): 415-26.
[16] YANG Z, WEI B, QIAO A, YANG P, CHEN W, ZHEN D, et al. A novel EZH2/NXPH4/CDKN2A axis is involved in regulating the proliferation and migration of non-small cell lung cancer cells [J]. Biosci Biotechnol Biochem, 2022, 86(3): 340-50.
[17] CHEN J, HONG J H, HUANG Y, LIU S, YIN J, DENG P, et al. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer [J]. Mol Cancer, 2023, 22(1): 85.
[18] PAWLYN C, BRIGHT M D, BUROS A F, STEIN C K, WALTERS Z, ARONSON L I, et al. Overexpression of EZH2 in multiple myeloma is associated with poor prognosis and dysregulation of cell cycle control [J]. Blood Cancer J, 2017, 7(3): e549.
[19] XU X, GU J, DING X, GE G, ZANG X, JI R, et al. LINC00978 promotes the progression of hepatocellular carcinoma by regulating EZH2-mediated silencing of p21 and E-cadherin expression [J]. Cell Death Dis, 2019, 10(10): 752.
[20] CHEN W M, CHEN W D, JIANG X M, JIA X F, WANG H M, ZHANG Q J, et al. HOX transcript antisense intergenic RNA represses E-cadherin expression by binding to EZH2 in gastric cancer [J]. World J Gastroenterol, 2017, 23(33): 6100-10.
[21] ZOU G, HUANG Y, ZHANG S, KO K P, KIM B, ZHANG J, et al. E-cadherin loss drives diffuse-type gastric tumorigenesis via EZH2-mediated reprogramming [J]. J Exp Med, 2024, 221(4).
[22] ZHUANG C, WANG P, HUANG D, XU L, WANG X, WANG L, et al. A double-negative feedback loop between EZH2 and miR-26a regulates tumor cell growth in hepatocellular carcinoma [J]. Int J Oncol, 2016, 48(3): 1195-204.
[23] BAI B, LIU Y, FU X M, QIN H Y, LI G K, WANG H C, et al. Dysregulation of EZH2/miR-138-5p Axis Contributes to Radiosensitivity in Hepatocellular Carcinoma Cell by Downregulating Hypoxia-Inducible Factor 1 Alpha (HIF-1α) [J]. Oxid Med Cell Longev, 2022, 2022: 7608712.
[24] ZHAO J, LI H, ZHAO S, WANG E, ZHU J, FENG D, et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling [J]. Mol Cancer, 2021, 20(1): 46.
[25] DENG M, WANG Z, LUO J, CAO H, LI Y, CHEN L, et al. CircZNF367 promotes osteoclast differentiation and osteoporosis by interacting with FUS to maintain CRY2 mRNA stability [J]. J Orthop Surg Res, 2023, 18(1): 492.
[26] WANG S, CHEN J, LI P, CHEN Y. LINC01133 can induce acquired ferroptosis resistance by enhancing the FSP1 mRNA stability through forming the LINC01133-FUS-FSP1 complex [J]. Cell Death Dis, 2023, 14(11): 767.
[27] XING C Y, ZHANG Y Z, HU W, ZHAO L Y. LINC00313 facilitates osteosarcoma carcinogenesis and metastasis through enhancing EZH2 mRNA stability and EZH2-mediated silence of PTEN expression [J]. Cell Mol Life Sci, 2022, 79(7): 382.
[28] WU T, WANG G, ZENG X, SUN Z, LI S, WANG W, et al. Hsa_circ_0006232 promotes laryngeal squamous cell cancer progression through FUS-mediated EZH2 stabilization [J]. Cell Cycle, 2021, 20(18): 1799-811.
[29] DI W, WEINAN X, XIN L, ZHIWEI Y, XINYUE G, JINXUE T, et al. Long noncoding RNA SNHG14 facilitates colorectal cancer metastasis through targeting EZH2-regulated EPHA7 [J]. Cell Death Dis, 2019, 10(7): 514.
[30] SOUROULLAS G P, JECK W R, PARKER J S, SIMON J M, LIU J Y, PAULK J, et al. An oncogenic Ezh2 mutation induces tumors through global redistribution of histone 3 lysine 27 trimethylation [J]. Nat Med, 2016, 22(6): 632-40.
[31] SNEERINGER C J, SCOTT M P, KUNTZ K W, KNUTSON S K, POLLOCK R M, RICHON V M, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas [J]. Proc Natl Acad Sci U S A, 2010, 107(49): 20980-5.
[32] SCORE J, HIDALGO-CURTIS C, JONES A V, WINKELMANN N, SKINNER A, WARD D, et al. Inactivation of polycomb repressive complex 2 components in myeloproliferative and myelodysplastic/myeloproliferative neoplasms [J]. Blood, 2012, 119(5): 1208-13.
[33] ZHANG D, QIU Y, ZHANG W, DU D, LIU Y, LIU L, et al. Homeobox B9 promotes the invasion and metastasis of hepatocellular carcinoma cells via the EZH2-MIR203A-SNAI2 axis [J]. J Transl Med, 2024, 22(1): 918.
[34] ZHENG M, JIANG Y P, CHEN W, LI K D, LIU X, GAO S Y, et al. Snail and Slug collaborate on EMT and tumor metastasis through miR-101-mediated EZH2 axis in oral tongue squamous cell carcinoma [J]. Oncotarget, 2015, 6(9): 6797-810.
[35] LU L, LUO F, LIU Y, LIU X, SHI L, LU X, et al. Posttranscriptional silencing of the lncRNA MALAT1 by miR-217 inhibits the epithelial-mesenchymal transition via enhancer of zeste homolog 2 in the malignant transformation of HBE cells induced by cigarette smoke extract [J]. Toxicol Appl Pharmacol, 2015, 289(2): 276-85.
[36] ZHANG K, FANG T, SHAO Y, WU Y. TGF-β-MTA1-SMAD7-SMAD3-SOX4-EZH2 Signaling Axis Promotes Viability, Migration, Invasion and EMT of Hepatocellular Carcinoma Cells [J]. Cancer Manag Res, 2021, 13: 7087-99.
[37] ZANG C, NIE F Q, WANG Q, SUN M, LI W, HE J, et al. Long non-coding RNA LINC01133 represses KLF2, P21 and E-cadherin transcription through binding with EZH2, LSD1 in non small cell lung cancer [J]. Oncotarget, 2016, 7(10): 11696-707.
[38] ZHANG S, LIAO W, WU Q, HUANG X, PAN Z, CHEN W, et al. LINC00152 upregulates ZEB1 expression and enhances epithelial-mesenchymal transition and oxaliplatin resistance in esophageal cancer by interacting with EZH2 [J]. Cancer Cell Int, 2020, 20(1): 569.
[39] LIN Y H, WU M H, LIU Y C, LYU P C, YEH C T, LIN K H. LINC01348 suppresses hepatocellular carcinoma metastasis through inhibition of SF3B3-mediated EZH2 pre-mRNA splicing [J]. Oncogene, 2021, 40(28): 4675-85.
[40] VERMA A, SINGH A, SINGH M P, NENGROO M A, SAINI K K, SATRUSAL S R, et al. EZH2-H3K27me3 mediated KRT14 upregulation promotes TNBC peritoneal metastasis [J]. Nat Commun, 2022, 13(1): 7344.
[41] CARLSON S M, GOZANI O. Emerging technologies to map the protein methylome [J]. J Mol Biol, 2014, 426(20): 3350-62.
[42] LIU F, MA F, WANG Y, HAO L, ZENG H, JIA C, et al. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis [J]. Nat Cell Biol, 2017, 19(11): 1358-70.
[43] PAN T, HAO J, WANG Y, DUAN W, YUE L, GAO X. Role in post-translational modification of M2-type pyruvate kinase in tumorigenesis and development [J]. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 2023, 48(9): 1359-67.
[44] HUANG H, OUYANG Q, MEI K, LIU T, SUN Q, LIU W, et al. Acetylation of SCFD1 regulates SNARE complex formation and autophagosome-lysosome fusion [J]. Autophagy, 2023, 19(1): 189-203.
[45] YI F, CAI C, RUAN B, HAO M, YEO S K, HAAS M, et al. Regulation of RB1CC1/FIP200 stability and autophagy function by CREBBP-mediated acetylation in an intrinsically disordered region [J]. Autophagy, 2023, 19(6): 1662-77.
[46] HUANG C, HU F, SONG D, SUN X, LIU A, WU Q, et al. EZH2-triggered methylation of SMAD3 promotes its activation and tumor metastasis [J]. J Clin Invest, 2022, 132(5).
[47] HU X, LIU Y, SHEN H, ZHANG T, LIANG T. MTF2 facilitates the advancement of osteosarcoma through mediating EZH2/SFRP1/Wnt signaling [J]. J Orthop Surg Res, 2024, 19(1): 467.
[48] CHEN W, WANG H T, JI J F, WANG Z Y, SHI T, WU M H, et al. Epigenetic network of EZH2/SFRP1/Wnt in the epithelial-mesenchymal transition of laryngeal carcinoma cells [J]. Neoplasma, 2022, 69(3): 680-90.
[49] ZHANG Y, LIN C, LIAO G, LIU S, DING J, TANG F, et al. MicroRNA-506 suppresses tumor proliferation and metastasis in colon cancer by directly targeting the oncogene EZH2 [J]. Oncotarget, 2015, 6(32): 32586-601.
[50] LIANG G, HAN L, QU M, XUE J, XU D, WU X, et al. Down-regulation of EZH2 genes targeting RUNX3 affects proliferation, invasion, and metastasis of human colon cancer cells by Wnt/β-catenin signaling pathway [J]. Aging (Albany NY), 2023, 15(23): 13655-68.
[51] REN L, DENG H, JIANG Y, LIU C. Dual-Regulated Mechanism of EZH2 and KDM6A on SALL4 Modulates Tumor Progression via Wnt/β-Catenin Pathway in Gastric Cancer [J]. Dig Dis Sci, 2023, 68(4): 1292-305.
[53] ZINGG D, DEBBACHE J, PEñA-HERNáNDEZ R, ANTUNES A T, SCHAEFER S M, CHENG P F, et al. EZH2-Mediated Primary Cilium Deconstruction Drives Metastatic Melanoma Formation [J]. Cancer Cell, 2018, 34(1): 69-84.e14.
[54] XU H, ZHAO G, ZHANG Y, JIANG H, WANG W, ZHAO D, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/β-catenin signaling pathway by targeting EZH2 [J]. Stem Cell Res Ther, 2019, 10(1): 381.
[55] LIAN R, MA H, WU Z, ZHANG G, JIAO L, MIAO W, et al. EZH2 promotes cell proliferation by regulating the expression of RUNX3 in laryngeal carcinoma [J]. Mol Cell Biochem, 2018, 439(1-2): 35-43.
[56] CHEN Q, ZHENG P S, YANG W T. EZH2-mediated repression of GSK-3β and TP53 promotes Wnt/β-catenin signaling-dependent cell expansion in cervical carcinoma [J]. Oncotarget, 2016, 7(24): 36115-29.
[57] ZHANG J J, CHEN J T, HUA L, YAO K H, WANG C Y. miR-98 inhibits hepatocellular carcinoma cell proliferation via targeting EZH2 and suppressing Wnt/β-catenin signaling pathway [J]. Biomed Pharmacother, 2017, 85: 472-8.
[58] WANG Y F, YU L, HU Z L, FANG Y F, SHEN Y Y, SONG M F, et al. Regulation of CCL2 by EZH2 affects tumor-associated macrophages polarization and infiltration in breast cancer [J]. Cell Death Dis, 2022, 13(8): 748.
[59] ISSHIKI Y, CHEN X, TEATER M, KARAGIANNIDIS I, NAM H, CAI W, et al. EZH2 inhibition enhances T cell immunotherapies by inducing lymphoma immunogenicity and improving T cell function [J]. Cancer Cell, 2025, 43(1): 49-68.e9.
[60] PORAZZI P, NASON S, YANG Z, CARTURAN A, GHILARDI G, GURUPRASAD P, et al. EZH1/EZH2 inhibition enhances adoptive T cell immunotherapy against multiple cancer models [J]. Cancer Cell, 2025, 43(3): 537-51.e7.
[61] XIE Y, LIN H, WEI W, KONG Y, FANG Q, CHEN E, et al. LINC00839 promotes malignancy of liver cancer via binding FMNL2 under hypoxia [J]. Sci Rep, 2022, 12(1): 18757.
[62] HUANG Z, TANG Y, ZHANG J, HUANG J, CHENG R, GUO Y, et al. Hypoxia makes EZH2 inhibitor not easy-advances of crosstalk between HIF and EZH2 [J]. Life Metab, 2024, 3(4).
[63] HWANG-VERSLUES W W, CHANG P H, JENG Y M, KUO W H, CHIANG P H, CHANG Y C, et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy [J]. Proc Natl Acad Sci U S A, 2013, 110(30): 12331-6.
[64] SHAN L, ZHOU X, LIU X, WANG Y, SU D, HOU Y, et al. FOXK2 Elicits Massive Transcription Repression and Suppresses the Hypoxic Response and Breast Cancer Carcinogenesis [J]. Cancer Cell, 2016, 30(5): 708-22.
[65] SUN J, CAI X, YUNG M M, ZHOU W, LI J, ZHANG Y, et al. miR-137 mediates the functional link between c-Myc and EZH2 that regulates cisplatin resistance in ovarian cancer [J]. Oncogene, 2019, 38(4): 564-80.
[66] XU S, LI X, LI L, WANG Y, GENG C, GUO F, et al. CTCF-silenced miR-137 contributes to EMT and radioresistance in esophageal squamous cell carcinoma [J]. Cancer Cell Int, 2021, 21(1): 155.
[67] LIU H, LI W, YU X, GAO F, DUAN Z, MA X, et al. EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis [J]. Oncotarget, 2016, 7(35): 56338-54.
[68] ZHONG J, YANG X, CHEN J, HE K, GAO X, WU X, et al. Circular EZH2-encoded EZH2-92aa mediates immune evasion in glioblastoma via inhibition of surface NKG2D ligands [J]. Nat Commun, 2022, 13(1): 4795.
[69] LIU J, YANG L, LIU X, LIU L, LIU M, FENG X, et al. lncRNA HOTTIP Recruits EZH2 to Inhibit PTEN Expression and Participates in IM Resistance in Chronic Myeloid Leukemia [J]. Stem Cells Int, 2022, 2022: 9993393.
[70] HAYWARD N K, WILMOTT J S, WADDELL N, JOHANSSON P A, FIELD M A, NONES K, et al. Whole-genome landscapes of major melanoma subtypes [J]. Nature, 2017, 545(7653): 175-80.
[71] HODIS E, WATSON I R, KRYUKOV G V, AROLD S T, IMIELINSKI M, THEURILLAT J P, et al. A landscape of driver mutations in melanoma [J]. Cell, 2012, 150(2): 251-63.
[72] SINI M C, DONEDDU V, PALIOGIANNIS P, CASULA M, COLOMBINO M, MANCA A, et al. Genetic alterations in main candidate genes during melanoma progression [J]. Oncotarget, 2018, 9(9): 8531-41.
[73] TANDA E T, VANNI I, BOUTROS A, ANDREOTTI V, BRUNO W, GHIORZO P, et al. Current State of Target Treatment in BRAF Mutated Melanoma [J]. Front Mol Biosci, 2020, 7: 154.
[74] ZHANG Y, DU X L, WANG C J, LIN D C, RUAN X, FENG Y B, et al. Reciprocal activation between PLK1 and Stat3 contributes to survival and proliferation of esophageal cancer cells [J]. Gastroenterology, 2012, 142(3): 521-30.e3.
[75] UEBEL A, KEWITZ-HEMPEL S, WILLSCHER E, GEBHARDT K, SUNDERKöTTER C, GERLOFF D. Resistance to BRAF Inhibitors: EZH2 and Its Downstream Targets as Potential Therapeutic Options in Melanoma [J]. Int J Mol Sci, 2023, 24(3).
[76] YANG C, GU Y, GE Z, SONG C. Targeting EZH2 Promotes Chemosensitivity of BCL-2 Inhibitor through Suppressing PI3K and c-KIT Signaling in Acute Myeloid Leukemia [J]. Int J Mol Sci, 2022, 23(19).
[77] WANG X, XIE Z, LOU Z, CHEN Y, HUANG S, REN Y, et al. Regulation of the PTEN/PI3K/AKT pathway in RCC using the active compounds of natural products in vitro [J]. Mol Med Rep, 2021, 24(5).
[78] SU Y, WANG B, HUANG J, HUANG M, LIN T. YTHDC1 positively regulates PTEN expression and plays a critical role in cisplatin resistance of bladder cancer [J]. Cell Prolif, 2023, 56(7): e13404.
[79] DENG X, KONG F, LI S, JIANG H, DONG L, XU X, et al. A KLF4/PiHL/EZH2/HMGA2 regulatory axis and its function in promoting oxaliplatin-resistance of colorectal cancer [J]. Cell Death Dis, 2021, 12(5): 485.
[80] VESCHI V, VERONA F, THIELE C J. Cancer Stem Cells and Neuroblastoma: Characteristics and Therapeutic Targeting Options [J]. Front Endocrinol (Lausanne), 2019, 10: 782.
[81] KAMIJO T. Role of stemness-related molecules in neuroblastoma [J]. Pediatr Res, 2012, 71(4 Pt 2): 511-5.
[82] ADHIKARY G, GRUN D, BALASUBRAMANIAN S, KERR C, HUANG J M, ECKERT R L. Survival of skin cancer stem cells requires the Ezh2 polycomb group protein [J]. Carcinogenesis, 2015, 36(7): 800-10.
[83] EL BABA R, PASQUEREAU S, HAIDAR AHMAD S, DIAB-ASSAF M, HERBEIN G. Oncogenic and Stemness Signatures of the High-Risk HCMV Strains in Breast Cancer Progression [J]. Cancers (Basel), 2022, 14(17).
[84] LIU H, SUN Q, SUN Y, ZHANG J, YUAN H, PANG S, et al. MELK and EZH2 Cooperate to Regulate Medulloblastoma Cancer Stem-like Cell Proliferation and Differentiation [J]. Mol Cancer Res, 2017, 15(9): 1275-86.
[85] CHEN L, ZHANG M, FANG L, YANG X, CAO N, XU L, et al. Coordinated regulation of the ribosome and proteasome by PRMT1 in the maintenance of neural stemness in cancer cells and neural stem cells [J]. J Biol Chem, 2021, 297(5): 101275.
[86] VAN VLERKEN L E, KIEFER C M, MOREHOUSE C, LI Y, GROVES C, WILSON S D, et al. EZH2 is required for breast and pancreatic cancer stem cell maintenance and can be used as a functional cancer stem cell reporter [J]. Stem Cells Transl Med, 2013, 2(1): 43-52.
[87] SUVà M L, RIGGI N, JANISZEWSKA M, RADOVANOVIC I, PROVERO P, STEHLE J C, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance [J]. Cancer Res, 2009, 69(24): 9211-8.
[88] ZONG X, NEPHEW K P. Ovarian Cancer Stem Cells: Role in Metastasis and Opportunity for Therapeutic Targeting [J]. Cancers (Basel), 2019, 11(7).
[89] GORODETSKA I, LUKIYANCHUK V, PEITZSCH C, KOZERETSKA I, DUBROVSKA A. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype [J]. Int J Cancer, 2019, 145(11): 2974-85.
[90] CHEN Z, TANG W, ZHOU Y, HE Z. The role of LINC01419 in regulating the cell stemness in lung adenocarcinoma through recruiting EZH2 and regulating FBP1 expression [J]. Biol Direct, 2022, 17(1): 23.
[91] TSENG C F, CHEN L T, WANG H D, LIU Y H, SHIAH S G. Transcriptional suppression of Dicer by HOXB-AS3/EZH2 complex dictates sorafenib resistance and cancer stemness [J]. Cancer Sci, 2022, 113(5): 1601-12.
[92] ZHANG Y, TANG B, SONG J, YU S, LI Y, SU H, et al. Lnc-PDZD7 contributes to stemness properties and chemosensitivity in hepatocellular carcinoma through EZH2-mediated ATOH8 transcriptional repression [J]. J Exp Clin Cancer Res, 2019, 38(1): 92.
[93] LIN H, XIE Y, KONG Y, YANG L, LI M. Identification of Two Molecular Subtypes of Hepatocellular Carcinoma Based on Dysregulated Immune LncRNAs [J]. Front Mol Biosci, 2021, 8: 625858.
[94] CAI L, LIU Y, LI Y, LIU B, CAO Y, YANG W, et al. TRIM37 interacts with EZH2 to epigenetically suppress PTCH1 and regulate stemness in glioma stem cells through sonic hedgehog pathway [J]. J Neurooncol, 2024, 169(2): 269-79.
[95] MARIE S K, SHINJO S M. Metabolism and brain cancer [J]. Clinics (Sao Paulo), 2011, 66 Suppl 1(Suppl 1): 33-43.
[96] LIU Q, WANG L, WANG Z, YANG Y, TIAN J, LIU G, et al. GRIM-19 opposes reprogramming of glioblastoma cell metabolism via HIF1α destabilization [J]. Carcinogenesis, 2013, 34(8): 1728-36.
[97] ZHANG T, GONG Y, MENG H, LI C, XUE L. Symphony of epigenetic and metabolic regulation-interaction between the histone methyltransferase EZH2 and metabolism of tumor [J]. Clin Epigenetics, 2020, 12(1): 72.
[98] PASCAL L E, AI J, RIGATTI L H, LIPTON A K, XIAO W, GNARRA J R, et al. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate [J]. Angiogenesis, 2011, 14(3): 331-43.
[99] CHEN F, CHEN J, YANG L, LIU J, ZHANG X, ZHANG Y, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells [J]. Nat Cell Biol, 2019, 21(4): 498-510.
[100] PANG B, ZHENG X R, TIAN J X, GAO T H, GU G Y, ZHANG R, et al. EZH2 promotes metabolic reprogramming in glioblastomas through epigenetic repression of EAF2-HIF1α signaling [J]. Oncotarget, 2016, 7(29): 45134-43.
[101] WANG Y, WANG M, WEI W, HAN D, CHEN X, HU Q, et al. Disruption of the EZH2/miRNA/β-catenin signaling suppresses aerobic glycolysis in glioma [J]. Oncotarget, 2016, 7(31): 49450-8.
[102] ZHOU J, LIN Y, KANG X, LIU Z, ZOU J, XU F. Hypoxia-mediated promotion of glucose metabolism in non-small cell lung cancer correlates with activation of the EZH2/FBXL7/PFKFB4 axis [J]. Cell Death Dis, 2023, 14(5): 326.
[103] WANG J, YANG C, XU H, FAN X, JIA L, DU Y, et al. The Interplay Between HIF-1α and EZH2 in Lung Cancer and Dual-Targeted Drug Therapy [J]. Adv Sci (Weinh), 2024, 11(7): e2303904.
[104] VANTAKU V, PUTLURI V, BADER D A, MAITY S, MA J, ARNOLD J M, et al. Epigenetic loss of AOX1 expression via EZH2 leads to metabolic deregulations and promotes bladder cancer progression [J]. Oncogene, 2020, 39(40): 6265-85.
[105] GAN L, LI Q, NIE W, ZHANG Y, JIANG H, TAN C, et al. PROX1-mediated epigenetic silencing of SIRT3 contributes to proliferation and glucose metabolism in colorectal cancer [J]. Int J Biol Sci, 2023, 19(1): 50-65.
[106] CURRIE E, SCHULZE A, ZECHNER R, WALTHER T C, FARESE R V, JR. Cellular fatty acid metabolism and cancer [J]. Cell Metab, 2013, 18(2): 153-61.
[107] AHMAD F, PATRICK S, SHEIKH T, SHARMA V, PATHAK P, MALGULWAR P B, et al. Telomerase reverse transcriptase (TERT) - enhancer of zeste homolog 2 (EZH2) network regulates lipid metabolism and DNA damage responses in glioblastoma [J]. J Neurochem, 2017, 143(6): 671-83.
[108] YIEW N K H, GREENWAY C, ZARZOUR A, AHMADIEH S, GOO B, KIM D, et al. Enhancer of zeste homolog 2 (EZH2) regulates adipocyte lipid metabolism independent of adipogenic differentiation: Role of apolipoprotein E [J]. J Biol Chem, 2019, 294(21): 8577-91.
[109] HAYDEN A, JOHNSON P W, PACKHAM G, CRABB S J. S-adenosylhomocysteine hydrolase inhibition by 3-deazaneplanocin A analogues induces anti-cancer effects in breast cancer cell lines and synergy with both histone deacetylase and HER2 inhibition [J]. Breast Cancer Res Treat, 2011, 127(1): 109-19.
[110] YANG Y, YANG T, ZHAO Z, ZHANG H, YUAN P, WANG G, et al. Down-regulation of BMAL1 by MiR-494-3p Promotes Hepatocellular Carcinoma Growth and Metastasis by Increasing GPAM-mediated Lipid Biosynthesis [J]. Int J Biol Sci, 2022, 18(16): 6129-44.
[111] ZHANG Y, WU M J, LU W C, LI Y C, CHANG C J, YANG J Y. Metabolic switch regulates lineage plasticity and induces synthetic lethality in triple-negative breast cancer [J]. Cell Metab, 2024, 36(1): 193-208.e8.
[112] BAKHTIARI M, JORDAN S C, MUMME H L, SHARMA R, SHANMUGAM M, BHASIN S S, et al. ARMH1 is a novel marker associated with poor pediatric AML outcomes that affect the fatty acid synthesis and cell cycle pathways [J]. Front Oncol, 2024, 14: 1445173.
[113] SAHU U, MULLARKEY M P, MURPHY S A, ANDERSON J C, PUTLURI V, KAMAL A H M, et al. IDH status dictates oHSV mediated metabolic reprogramming affecting anti-tumor immunity [J]. Nat Commun, 2025, 16(1): 3874.
[114] ZHANG T, GUO Z, HUO X, GONG Y, LI C, HUANG J, et al. Dysregulated lipid metabolism blunts the sensitivity of cancer cells to EZH2 inhibitor [J]. EBioMedicine, 2022, 77: 103872.
[115] NOCITO M C, HANTEL C, LERARIO A M, MASTROROCCO F, DE MARTINO L, MUSICCO C, et al. A targetable antioxidant defense mechanism to EZH2 inhibitors enhances tumor cell vulnerability to ferroptosis [J]. Cell Death Dis, 2025, 16(1): 291.
[116] LIU Y, TU C E, GUO X, WU C, GU C, LAI Q, et al. Tumor-suppressive function of EZH2 is through inhibiting glutaminase [J]. Cell Death Dis, 2021, 12(11): 975.
[117] PAPATHANASSIU A E, KO J H, IMPRIALOU M, BAGNATI M, SRIVASTAVA P K, VU H A, et al. BCAT1 controls metabolic reprogramming in activated human macrophages and is associated with inflammatory diseases [J]. Nat Commun, 2017, 8: 16040.
[119] T W M F, ISLAM J M M, HIGASHI R M, LIN P, BRAINSON C F, LANE A N. Metabolic reprogramming driven by EZH2 inhibition depends on cell-matrix interactions [J]. J Biol Chem, 2024, 300(1): 105485.
[120] CANTLEY L C. The phosphoinositide 3-kinase pathway [J]. Science, 2002, 296(5573): 1655-7.
[121] WANG X Q, SUN P, PALLER A S. Inhibition of integrin-linked kinase/protein kinase B/Akt signaling: mechanism for ganglioside-induced apoptosis [J]. J Biol Chem, 2001, 276(48): 44504-11.
[122] LI Y, SONG Y H, MOHLER J, DELAFONTAINE P. ANG II induces apoptosis of human vascular smooth muscle via extrinsic pathway involving inhibition of Akt phosphorylation and increased FasL expression [J]. Am J Physiol Heart Circ Physiol, 2006, 290(5): H2116-23.
[123] TIAN Y, CHEN Z H, WU P, ZHANG D, MA Y, LIU X F, et al. MIR497HG-Derived miR-195 and miR-497 Mediate Tamoxifen Resistance via PI3K/AKT Signaling in Breast Cancer [J]. Adv Sci (Weinh), 2023, 10(12): e2204819.
[124] LI H, SHEN X, MA M, LIU W, YANG W, WANG P, et al. ZIP10 drives osteosarcoma proliferation and chemoresistance through ITGA10-mediated activation of the PI3K/AKT pathway [J]. J Exp Clin Cancer Res, 2021, 40(1): 340.
[125] KONG C, WU M, LU Q, KE B, XIE J, LI A. PI3K/AKT confers intrinsic and acquired resistance to pirtobrutinib in chronic lymphocytic leukemia [J]. Leuk Res, 2024, 144: 107548.
[126] SANCHES J G P, SONG B, ZHANG Q, CUI X, YABASIN I B, NTIM M, et al. The Role of KDM2B and EZH2 in Regulating the Stemness in Colorectal Cancer Through the PI3K/AKT Pathway [J]. Front Oncol, 2021, 11: 637298.
[127] DAI Q, ZHANG T, PAN J, LI C. LncRNA UCA1 promotes cisplatin resistance in gastric cancer via recruiting EZH2 and activating PI3K/AKT pathway [J]. J Cancer, 2020, 11(13): 3882-92.
[128] CHEN J, WANG F, XU H, XU L, CHEN D, WANG J, et al. Long Non-Coding RNA SNHG1 Regulates the Wnt/β-Catenin and PI3K/AKT/mTOR Signaling Pathways via EZH2 to Affect the Proliferation, Apoptosis, and Autophagy of Prostate Cancer Cell [J]. Front Oncol, 2020, 10: 552907.
[129] XU K, WU Z J, GRONER A C, HE H H, CAI C, LIS R T, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent [J]. Science, 2012, 338(6113): 1465-9.
[130] WEI X, GUO J, LI Q, JIA Q, JING Q, LI Y, et al. Bach1 regulates self-renewal and impedes mesendodermal differentiation of human embryonic stem cells [J]. Sci Adv, 2019, 5(3): eaau7887.
[131] BASU S, CHERIYAMUNDATH S, BEN-ZE'EV A. Cell-cell adhesion: linking Wnt/β-catenin signaling with partial EMT and stemness traits in tumorigenesis [J]. F1000Res, 2018, 7.
[132] CAO W, LEE H, WU W, ZAMAN A, MCCORKLE S, YAN M, et al. Multi-faceted epigenetic dysregulation of gene expression promotes esophageal squamous cell carcinoma [J]. Nat Commun, 2020, 11(1): 3675.
[133] FAZI B, GARBO S, TOSCHI N, MANGIOLA A, LOMBARI M, SICARI D, et al. The lncRNA H19 positively affects the tumorigenic properties of glioblastoma cells and contributes to NKD1 repression through the recruitment of EZH2 on its promoter [J]. Oncotarget, 2018, 9(21): 15512-25.
[134] KHAN H, NI Z, FENG H, XING Y, WU X, HUANG D, et al. Combination of curcumin with N-n-butyl haloperidol iodide inhibits hepatocellular carcinoma malignant proliferation by downregulating enhancer of zeste homolog 2 (EZH2) - lncRNA H19 to silence Wnt/β-catenin signaling [J]. Phytomedicine, 2021, 91: 153706.
[135] YU T, ZHOU F, TIAN W, XU R, WANG B, ZENG A, et al. EZH2 interacts with HP1BP3 to epigenetically activate WNT7B that promotes temozolomide resistance in glioblastoma [J]. Oncogene, 2023, 42(6): 461-70.
[136] JUNG H Y, JUN S, LEE M, KIM H C, WANG X, JI H, et al. PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation [J]. Mol Cell, 2013, 52(2): 193-205.
[137] YU D, WANG S, WANG J, ZHANG K, NIU Z, LIN N. EZH2-STAT3 signaling pathway regulates GSDMD-mediated pyroptosis in glioblastoma [J]. Cell Death Discov, 2024, 10(1): 341.
[138] ZHANG D, YANG X J, LUO Q D, FU D L, LI H L, LI H C, et al. EZH2 enhances the invasive capability of renal cell carcinoma cells via activation of STAT3 [J]. Mol Med Rep, 2018, 17(3): 3621-6.
[139] LI L, ZHANG Y, YANG K, LIU W, ZHOU Z, XU Y. miRNA-449c-5p regulates the JAK-STAT pathway in inhibiting cell proliferation and invasion in human breast cancer cells by targeting ERBB2 [J]. Cancer Rep (Hoboken), 2024, 7(2): e1974.
[140] SAKAHARA M, OKAMOTO T, OYANAGI J, TAKANO H, NATSUME Y, YAMANAKA H, et al. IFN/STAT signaling controls tumorigenesis and the drug response in colorectal cancer [J]. Cancer Sci, 2019, 110(4): 1293-305.
[141] ZHENG M, CAO M X, LUO X J, LI L, WANG K, WANG S S, et al. EZH2 promotes invasion and tumour glycolysis by regulating STAT3 and FoxO1 signalling in human OSCC cells [J]. J Cell Mol Med, 2019, 23(10): 6942-54.
[142] GU C J, XIE F, ZHANG B, YANG H L, CHENG J, HE Y Y, et al. High Glucose Promotes Epithelial-Mesenchymal Transition of Uterus Endometrial Cancer Cells by Increasing ER/GLUT4-Mediated VEGF Secretion [J]. Cell Physiol Biochem, 2018, 50(2): 706-20.
[143] XU Q, ZHANG Q, ISHIDA Y, HAJJAR S, TANG X, SHI H, et al. EGF induces epithelial-mesenchymal transition and cancer stem-like cell properties in human oral cancer cells via promoting Warburg effect [J]. Oncotarget, 2017, 8(6): 9557-71.
[144] LI C, SONG J, GUO Z, GONG Y, ZHANG T, HUANG J, et al. EZH2 Inhibitors Suppress Colorectal Cancer by Regulating Macrophage Polarization in the Tumor Microenvironment [J]. Front Immunol, 2022, 13: 857808.
[145] ZHOU X, CHEN H, HU Y, MA X, LI J, SHI Y, et al. Enhancer of zeste homolog 2 promotes renal fibrosis after acute kidney injury by inducing epithelial-mesenchymal transition and activation of M2 macrophage polarization [J]. Cell Death Dis, 2023, 14(4): 253.
[146] YANG Q, ZHAO S, SHI Z, CAO L, LIU J, PAN T, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling [J]. J Exp Clin Cancer Res, 2021, 40(1): 120.
[147] FUJII S, TOKITA K, WADA N, ITO K, YAMAUCHI C, ITO Y, et al. MEK-ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes [J]. Oncogene, 2011, 30(39): 4118-28.
[148] CAI Y, CHEN P, XU H, LI S, ZHAO B, FAN Y, et al. EZH2 Gene Knockdown Inhibits Sheep Pituitary Cell Proliferation via Downregulating the AKT/ERK Signaling Pathway [J]. Int J Mol Sci, 2023, 24(13).
[149] ZHANG Q, SHI Y, LIU S, YANG W, CHEN H, GUO N, et al. EZH2/G9a interact to mediate drug resistance in non-small-cell lung cancer by regulating the SMAD4/ERK/c-Myc signaling axis [J]. Cell Rep, 2024, 43(2): 113714.
[150] HOXHA S, SHEPARD A, TROUTMAN S, DIAO H, DOHERTY J R, JANISZEWSKA M, et al. YAP-Mediated Recruitment of YY1 and EZH2 Represses Transcription of Key Cell-Cycle Regulators [J]. Cancer Res, 2020, 80(12): 2512-22.
[151] LO SARDO F, TURCO C, MESSINA B, SACCONI A, AUCIELLO F R, PULITO C, et al. The oncogenic axis YAP/MYC/EZH2 impairs PTEN tumor suppression activity enhancing lung tumorigenicity [J]. Cell Death Discov, 2024, 10(1): 452.
[152] LUO C, BALSA E, PERRY E A, LIANG J, TAVARES C D, VAZQUEZ F, et al. H3K27me3-mediated PGC1α gene silencing promotes melanoma invasion through WNT5A and YAP [J]. J Clin Invest, 2020, 130(2): 853-62.
[153] JEONG G Y, PARK M K, CHOI H J, AN H W, PARK Y U, CHOI H J, et al. NSD3-Induced Methylation of H3K36 Activates NOTCH Signaling to Drive Breast Tumor Initiation and Metastatic Progression [J]. Cancer Res, 2021, 81(1): 77-90.
[154] QIAN X, ZHANG Y. EZH2 enhances proliferation and migration of trophoblast cell lines by blocking GADD45A-mediated p38/MAPK signaling pathway [J]. Bioengineered, 2022, 13(5): 12583-97.

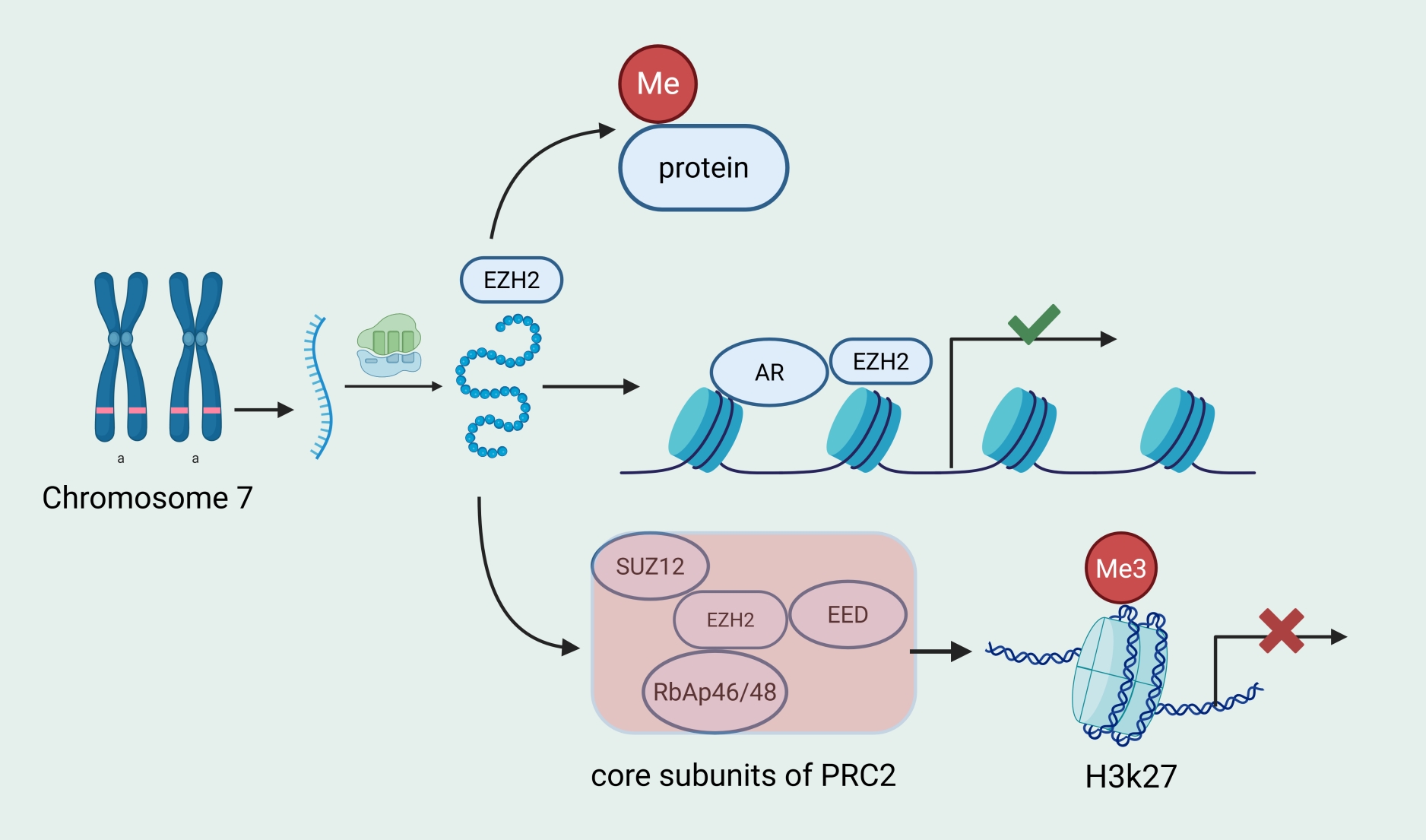

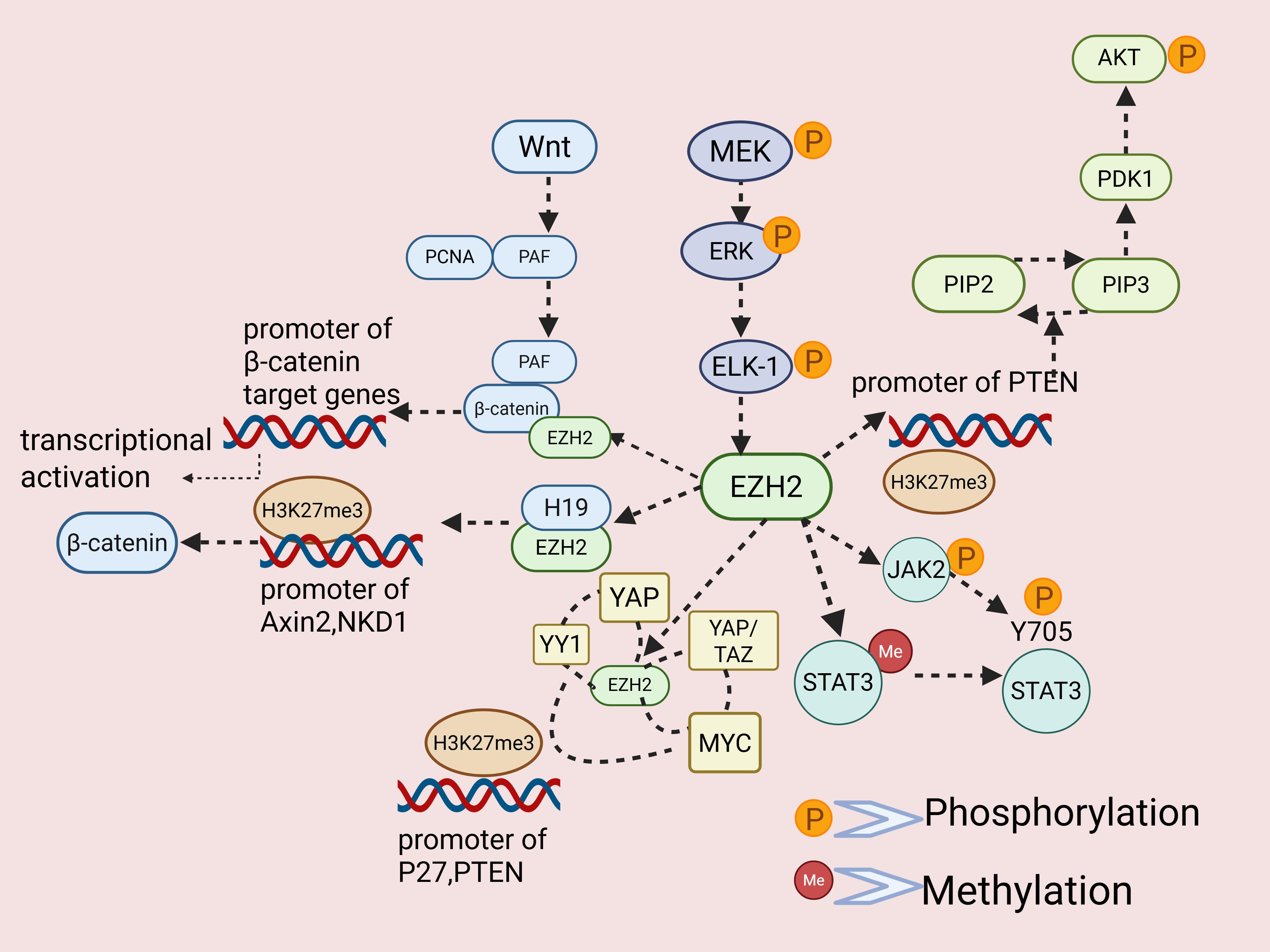
 Figures
Figures References
References Peer
Peer Information
Information




