Abstract

Necrotizing fasciitis (NF) is a life-threatening soft tissue infection typically associated with marine pathogens such as Vibrio vulnificus in cases involving fish-related injuries. This case report describes a rare instance of NF caused by Aeromonas sobria following a freshwater fish (Chinese carp) injury in a 58-year-old immunocompromised woman with a 20-year history of rheumatoid arthritis on long-term immunosuppressive therapy. The patient presented with rapid-onset swelling, pain, and septic shock, requiring intensive care, vasopressor support, and four surgical debridements with negative pressure wound therapy. Wound cultures confirmed Aeromonas sobria, contrasting with the more common marine-acquired Vibrio infections. Despite delayed diagnosis, aggressive multimodal management—including broad-spectrum antibiotics (piperacillin-tazobactam followed by meropenem) and repeated surgical interventions—resulted in survival after 41 days of hospitalization. This case highlights the importance of considering freshwater pathogens in NF, particularly in immunocompromised patients, and underscores the critical role of early surgical debridement and empiric antimicrobial coverage for atypical organisms. Clinicians should maintain a high suspicion for NF in fish-related injuries, even without marine exposure, to prevent fatal outcomes.
Keywords: Necrotizing fasciitis, Aeromonas sobria, Immunocompromised host, Septic shock.
Introduction
Necrotizing fasciitis is one of the fatal skin and soft tissue infections. In the cases caused by fish wounds, most of them are caused by marine fish wounds, and Vibrio vulnificus has been detected in almost all cases [1-4]. Even when combined with a clear history of seafood exposure and a positive vibrio vulnificus culture, and most physicians give early warning and treat it with caution, the prognosis of necrotizing fasciitis is not optimistic [5]. In the absence of typical contact history, some patients are likely to be missed, resulting in delayed treatment. This paper reports a case of necrotizing fasciitis in our center, which was injured by freshwater fish and cultured as mild aeromonas, and summarizes relevant literature to provide reference experience for clinical treatment of similar diseases.
Case Presentation
The 58-year-old female patient had a history of rheumatoid arthritis for 20 years. She had been taking tripterygium wirelli for immunosuppressive therapy for a long time. She had a history of right knee fracture surgery and binocular cataract surgery. Nine days earlier, a freshwater fish (Chinese carp) had stabbed the area between the thumb and forefinger of the left hand, and the following day the patient experienced swelling and pain in the affected area. The patient removed the scab on her own and disinfected the wound with iodine povidone, but her condition did not improve and the swelling worsened, increasing to the whole palm and wrist, so she was admitted to the emergency department. On the day of the patient's visit, she presented with severe symptoms of unconsciousness and a drop in blood pressure (62/42 mmHg), with further swelling of the stabbed area. Emergency physicians immediately supplemented blood volume, applied vasoactive drugs and transferred the patient to the intensive care unit. Critical care physicians applied piperacillin-tazobactam for anti-infective treatment and cultured mild Aeromonas sp. in the wound secretions, and continued aggressive rehydration with high doses of vasoactive medications (norepinephrine 160ug/min to maintain blood pressure around 120/70mmHg) to maintain the patient's vital signs. The patient underwent a first emergency surgical debridement on the first day of hospitalization, during which the surgeon incised the affected area for thorough debridement and placed a Vacuum Sealing Drainage (VSD)(Figure1A-B). After the operation, the patient was returned to the intensive care unit, where mechanical ventilation was continued, followed by an anti-infective regimen of meropenem (1gQ8H), vasoactive drugs, gammaglobulin, allogeneic plasma, and albumin to maintain the patient's vital signs. On the 20th day of hospitalization, the patient's vital signs stabilized, and he was extubated and resumed spontaneous respiration. The patient underwent the second, third, and fourth surgeries on the 8th, 19th, and 26th days after hospitalization, respectively(Figure1C-F) . Finally, the patient was discharged from the hospital with a total of 41 days of hospitalization, including 27 days in the intensive care unit.
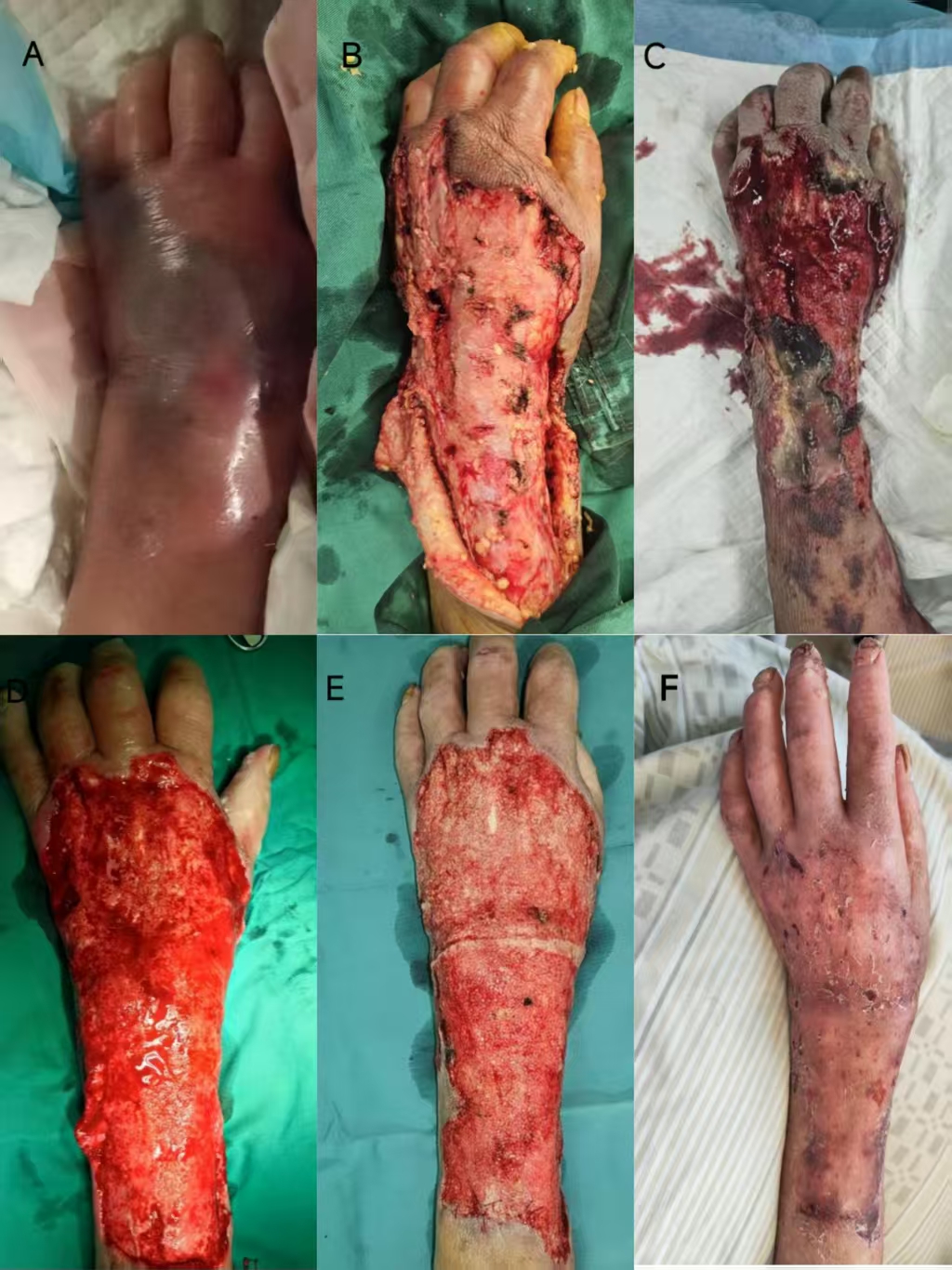
Figure 1. Wound Progression. (A) On emergency admission, the patient had significant swelling of the left hand and forearm. (B) Debridement was performed for the first time, and VSD suction was placed postoperatively. (C) After the first VSD, a large amount of necrotic tissue was still seen. (D) After the second VSD, the local infection was significantly improved, and the basal granulation growth was unsatisfactory. (E) After the third VSD, the granulation tissue grew satisfactorily and healthy skin was grafted to the granulation tissue. (F) The skin survived, and left hand function was preserved.
Discussion
Regarding the treatment of necrotizing fasciitis, there are no other than three problems: early diagnosis, antibacterial treatment and surgical intervention, but in practice all three issues are not easily resolved in clinical practice.
DiagnosisNecrotizing fasciitis is a life-threatening soft tissue infection characterized by rapidly progressive destruction of the muscle fascia and surrounding soft tissues. Depending on the infecting bacteria, necrotizing fasciitis is usually classified into four types [6]: Type I: infections involving multiple microorganisms; Type II: infections caused by a single pathogen, mainly hemolytic streptococci; Type III: infections involving Clostridium, Vibrio, and Gram-negative bacteria; and Type IV: infections involving fungi. The initial symptoms of necrotizing fasciitis are often non-specific, presenting only non-characteristic symptoms such as pain, redness, swelling, and erythema, making clinical diagnosis a challenge. However, when severe manifestations of necrotizing fasciitis occur, such as localized twisting, skin necrosis, or even infectious shock, it is often indicative of a progression of the infection. Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) is a clinical tool first described by Wong [7] et al. It is thought to be able to detect cases of necrotizing fasciitis in early clinical stages, but some scholars are skeptical about its practical application: the LRINEC score used to diagnose necrotizing fasciitis had a low Receiver Operating Characteristic Curve (ROC) in a study by Rav [8] et al. It is not recommended to be used alone and needs to be combined with history, physical examination and imaging; Chang [9] concluded that the accuracy of the LRINEC score is unreliable in necrotizing fasciitis involving Vibrio traumaticus.
Although the clinical symptoms of necrotizing fasciitis are similar to those of cellulitis and other soft-tissue infections in the initial stage, both of which manifest as erythema, localized fever, skin hardness and edema, necrotizing fasciitis often manifests itself as severe pain that is not proportional to the severity of the lesion [10]. And usually within two days, patients not only have more intense pain, but most of them develop systemic symptoms such as fever, confusion, diarrhea, nausea and vomiting, and after four days, patients may develop infectious shock or multi-organ failure, which includes hypotension, metabolic acidosis, coagulation disorders, and malnutrition [11]. In all cases with clinical symptoms similar to cellulitis, there is a need to be vigilant and a high suspicion of necrotizing fasciitis, especially when accompanied by severe pain that progresses rapidly and is not consistent with the condition. Sarani et al.[12] suggested local biopsy histopathology in highly suspicious cases to confirm the diagnosis of necrotizing fasciitis in an earlier study.
Medical imaging is a powerful tool in the diagnosis of necrotizing fasciitis. Although radiographs are less sensitive, they can also be used routinely as soft tissue gas can be observed in most case images of necrotizing fasciitis [13] and can rule out potential foreign bodies or fractures. Ultrasonography similarly lacks resolution of deeper structures and is therefore of limited diagnostic significance in necrotizing fasciitis. However, the visualization of fascial irregularities on ultrasound images, or abnormal fluid accumulation in deep fascial planes can also help to differentiate necrotizing fasciitis from cellulitis [14]. Computed tomography (CT) examination is the main imaging modality for necrotizing fasciitis due to its high spatial resolution, and the visualization of gas in the deep fascial planes on CT images is a diagnostic hallmark of necrotizing fasciitis [15], but it does not exclude the diagnosis of necrotizing fasciitis in some of the cases in which there is no soft-tissue gas [16]. Magnetic resonance imaging has an extremely high contrast resolution for the diagnosis of soft tissue infections and can achieve a sensitivity of 93% for the diagnosis of necrotizing fasciitis [17], which can be used as the gold standard for the diagnosis of necrotizing fasciitis. Thickening of the deep fascia and subfascial effusion observed on magnetic resonance imaging can suggest the diagnosis of necrotizing fasciitis, which can be confirmed when the thickness of the deep fascia is greater than 3 mm and is accompanied by the involvement of multiple fascial compartments.
Antimicrobial TherapyThe importance of rational antibiotic application for the treatment of necrotizing fasciitis cannot be overstated, but the clinical characteristics of necrotizing fasciitis also have special requirements for antibiotic application. Firstly, due to tissue hypoxia and ischemia at the site of infection, the blood is unable to deliver enough antibiotics to the site of infection [18], which means that a single antibiotic is of limited therapeutic value, and at the same time, most studies have shown that necrotizing fasciitis occurs mostly by a mixture of multiple bacterial infections [8.19.20], which calls for early and adequate combined application of broad-spectrum antibiotics in the treatment of necrotizing fasciitis. Due to the rapid onset of necrotizing fasciitis, its diagnosis and use of antibiotics cannot be completely dependent on bacterial cultures of samples, in the fatal case reported by Merola [21] et al. a 49 year old female patient with necrotizing fasciitis died of infectious shock after the initial bacterial cultures of samples were negative and the autopsy report showed the presence of Streptococcus pyogenes in the pleural and pericardial fluids. Thus the principle of antibiotic use in necrotizing fasciitis cannot be overemphasized.
Regarding the study of susceptible bacteria, Nazerani [22] et al. concluded that group A hemolytic streptococci were the most frequently detected microorganisms, while Molewa [23] et al. found that Staphylococcus aureus and Escherichia coli were the most prevalent gram-positive and gram-negative organisms, respectively, while they recommended amoxicillin clavulanate and clindamycin for empirical treatment. In the study of Guliyeva [24], similar findings were obtained that infection in necrotizing fasciitis was dominated by mixed bacterial infections, which accounted for 70-90% of all cases, with anaerobic bacteria being dominated by non-typable Streptococcus and Enterobacteriaceae bacteria, and aerobic bacteria being dominated by Staphylococcus aureus. In the choice of antibiotics, carbapenems (imipenem, meropenem, or ertapenem) or piperacillin-tazobactam plus vancomycin or daptomycin (good coverage for methicillin-resistant staphylococcus aureus (MRSA) ) plus clindamycin (for toxin-secreting strains of GAS and Staphylococcus aureus) are usually recommended in the empiric phase of treatment. After bacterial culture results are obtained and necrotizing fasciitis is more accurately typed, then ampicillin/ampicillin-sulbactam in combination with metronidazole or clindamycin is recommended for type I necrotizing fasciitis; for type II necrotizing fasciitis, a first- or second-generation cephalosporin is used for coverage of Streptococcus pyogenes and Staphylococcus aureus; for type III necrotizing fasciitis, clindamycin and penicillin; and for type IV patients, fungal therapy such as fluconazole is emphasized [24].
Surgical InterventionAs stated earlier, the use of antibiotics for necrotizing fasciitis cannot be overemphasized, but paradoxically, the use of antibiotics alone has a limited therapeutic effect on necrotizing fasciitis because bacteria can rapidly multiply and progress along the deep fascial layers, and due to the hypoxia and ischemia of the tissues, the antibiotics are not efficiently transported to the tissues [18], therefore, compared to the application of antibiotics, the surgical intervention becomes more indispensable.
The importance of surgical intervention has been emphasized in almost all studies involving necrotizing fascial strictures [8, 10, 12, 20, 21], with agreement that early debridement improves patient survival. In terms of surgical approach, negative pressure wound therapy has been recognized by an increasing number of researchers [25], and also, in this case, we applied this technique several times. Negative pressure technique was first proposed by Morykwas [26] et al. in 1997 and is believed to stabilize the wound environment, reduce wound edema, decrease bacterial load, improve tissue attention, and stimulate granulation tissue and angiogenesis. In comparison with conventional dressings, negative pressure vacuum therapy is thought to offer superiority in preventing hematoma formation and infection [27], as well as promoting healing of burn wounds [28]. Today this technique is widely used for orthopedic infections [29], diabetic ulcers [30], and patients with abscessed chests [31].However, we must emphasize that due to the potential risks associated with anaerobic bacteria, we must exercise caution when using negative pressure wound therapy on wounds where infection has not been fully controlled. We recommend that patients with stable vital signs undergo thorough debridement treatment before undergoing negative pressure wound therapy.
After successful radical debridement, the patient's vital signs often stabilize, and the next step of repair and reconstruction is also a major challenge for the surgeon. In this case, we have applied a fracture skin graft to repair the forearm defect, which was obtained from the healthy chest wall, which is practicable and convenient in the case of well-germinated defects, but in the case of complex defects, a simple skin graft would not be able to solve the problem. Lotus flaps with perforating vessels are a good alternative when there is a defect in the perineal area [32]. Artificial tissue substitutes have been favored by clinical surgeons in recent years, such as Mirzania [33] using umbilical amniotic tissue (umbilical amniotic tissue) for eyelid reconstruction after debridement for necrotizing fasciitis and Tobalem [34] applying NovoSorb biodegradable temporizing matrix) for extensive trunk reconstruction in children after necrotizing fasciitis debridement, both with good results.
This CaseImmunodeficiency is a common risk factor for necrotizing fasciitis, and other common risk factors include diabetes mellitus, obesity, alcoholism, chronic renal failure, and organ transplant patients [35]. The patient in this case was a female patient with a 20-year history of rheumatoid arthritis who was in a hypo-immune state due to long-term oral administration of tripterygium wilfordii (a Chinese medicine that regulates the immune system). The patient's lymphocyte count was consistently below 20%, which resulted in a higher systemic immune-inflammatory index (SII), and a higher SII index has been associated with lethal necrotizing fasciitis in previous studies [36]. This may explain the rapid progression of this patient's disease. No subcutaneous friction rub or radiographic evidence of gas accumulation was observed in this case. We made a diagnosis of necrotizing fasciitis based on surgical exploration. Initial emergency surgical exploration: A longitudinal incision was made along the dorsal aspect of the left forearm, revealing diffuse grayish-black deep fascia. Resistance disappeared during blunt dissection of the subcutaneous tissue and fascia layer (positive “finger test”), and thin, turbid fluid exuded from the muscle surface after fascial incision (positive “drip sign”). The necrotic fascia was resected to the edge of bleeding. During the second debridement surgery, we found that the residual necrotic fascia had clear boundaries (separated from the viable tissue), and there was no capillary bleeding at the wound site. A subsequent pus culture confirmed a single-bacterium infection by A. sobria. During the third surgery, new granulation tissue was observed covering the viable fascia in the original surgical area, and the remaining necrotic foci were thoroughly removed. During the fourth surgery, the skin graft bed exhibited granular granulation tissue with no exposure of deep tissue, and an autologous skin graft was performed on the wound. In previous cases of fish stab wounds, stab wounds from marine fish or exposure to contaminated seawater were predominant, whereas in the present case, the patient had no history of seafood exposure and was only partially stabbed by the fin of a freshwater fish, which in bacterial cultures showed the causative organism to be Aeromonas mildewii, which is also different from Vibrio traumaticus found in most fish stab wounds [4]. Aeromonas sobria, also belonging to the family of Vibrio (Vibrionaceae), is a commensal conditionally pathogenic bacterium in humans, fish, and animals [37], widely found in soil and water bodies and parasitized in aquatic animals, and is often reported to be associated with acute gastroenteritis and bacterial peritonitis in humans. However, this case suggests that Aeromonas mildans may cause fatal necrotizing fasciitis in immunocompromised conditions.In previous studies, some scholars have reported similar cases, although they are not entirely identical to ours. Hutchinson [38] reported a case of necrotizing fasciitis caused by lake water in 2021. A previously healthy 66-year-old woman sustained a deep laceration on the posterior aspect of her right lower leg, which was subsequently contaminated with lake water. After the wound was irrigated and sutured, the patient developed NF. Al Nour AH [39] reported a more severe case in 2024. They described a fatal retroperitoneal NF caused by Aeromonas caviae in a patient with a history of gastric cancer. Chang [40] reported a case of necrotizing fasciitis in a patient with neutropenia. Das [41] reported a case of necrotizing fasciitis in a recipient of allogeneic unrelated hematopoietic stem cell transplantation. Tsai [42] reported two cases of long-term diabetic patients who developed fatal necrotizing fasciitis due to infection with Aeromonas sobria. In reported cases [40-42] involving patients with immunodeficiency, the outcome of necrotizing fasciitis is often worse, and we believe that immunosuppression is central to its rapid progression.Therefore, we recommend that any fish sting injury in immunocompromised patients (regardless of freshwater/saltwater) should be considered a surgical emergency. For such patients, physicians should immediately administer intravenous antibiotics targeting Aeromonas hydrophila and Vibrio species (e.g., third-generation cephalosporins + ciprofloxacin) and assess the LRINEC score every 4 hours within 24 hours. If the patient's SII > 900 or C-Reactive Protein (CRP) > 150 mg/L, we recommend early debridement.
Due to the urgent need for life-saving measures, no tissue pathological evidence was obtained. In future similar cases, we recommend obtaining additional specimens during a second debridement procedure in the stable phase to further investigate the underlying mechanisms.
Conclusion
We highlight the need for vigilance regarding necrotizing fasciitis in patients with fish-related injuries, even without seafood exposure—particularly in immunocompromised individuals or those infected with Aeromonas hydrophila. Early recognition and aggressive surgical debridement are critical for successful treatment.
Abbreviations
C-Reactive Protein: CRP; Laboratory Risk Indicator for Necro-tizing Fasciitis: LRINEC; Methicillin-Resistant Staphylococcus Aureus: MRSA; Systemic Immune-inflammatory Index: SII; Vac-uum Sealing Drainage: VSD.
Declarations
Author contributions
Zhengnan Zhao, Peng Wang, Lei Ma and Jiabao Yang designed the whole project. Yang Wang, Jiaping Guo wrote the manu-script. Chenglong Yao, Zefan Sun and Xiu Wang contributed to the data collection. Jiabao Yang contributed to the methodolo-gy. All authors read and approved the final manuscript.
Acknowledgements
Not Applicable
Funding information
Not applicable.
Ethics approval and consent to participate
Ethical issues (Including plagiarism, informed consent, mis-conduct, data fabrication and/or falsification, double publica-tion and/or submission, redundancy, etc.) have been complete-ly observed by the authors.
Competing Interests
The authors declare that they have no competing interests.The authors declare that they have no existing or potential commercial or financial relationships that could create a con-flict of interest at the time of conducting this study.
Data availability
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. Addi-tional data related to this paper may be requested from the authors.
References
 Figures
Figures References
References Peer
Peer Information
InformationFigure 1. Wound Progression. (A) On emergency admission, the patient had significant swelling of the left hand and forearm. (B) Debridement was performed for the first time, and VSD suction was placed postoperatively. (C) After the first VSD, a large amount of necrotic tissue was still seen. (D) After the second VSD, the local infection was significantly improved, and the basal granulation growth was unsatisfactory. (E) After the third VSD, the granulation tissue grew satisfactorily and healthy skin was grafted to the granulation tissue. (F) The skin survived, and left hand function was preserved.

Peer-review Terminology
Identity transparency: Single anonymized
Reviewer interacts with: Editor
Review information published:
Review reports
Reviewer identities if reviewer opts in
Author/reviewer communication
Details
© 2025 The Author(s). Life Conflux published by Life Conflux Press Limited on behalf of Conflux Science.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Publication History
Received 2025-04-12
Accepted 2025-08-22
Published 2025-09-25


